Selective Carboxylate Recognition Using Urea-Functionalized Unclosed Cryptands: Mild Synthesis and Complexation Studies
- PMID: 32142280
- PMCID: PMC7497646
- DOI: 10.1021/acs.joc.9b03082
Selective Carboxylate Recognition Using Urea-Functionalized Unclosed Cryptands: Mild Synthesis and Complexation Studies
Abstract
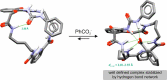
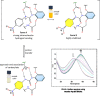
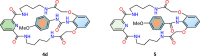
Herein we present the synthesis and evaluation of anion-binding properties of 12 new receptors from the unclosed cryptand family. Their core is built on the stable 26-membered tetraamidic macrocyclic scaffold, whereas various alkyl and aryl urea substituents were introduced after a yield-limiting macrocyclization step (65-98%). The receptors strongly bind anions, in particular carboxylates, even in a highly competitive solvent mixture (DMSO-d6 + H2O 95:5 v/v).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

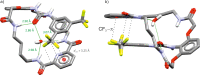
References
-
-
For examples, see:
- Dietrich B.; Lehn J. M.; Sauvage J. P. Diaza-Polyoxa-Macrocycles et Macrobicycles. Tetrahedron Lett. 1969, 10, 2885–2888. 10.1016/S0040-4039(01)88299-X. - DOI
- Dietrich B.; Lehn J. M.; Sauvage J. P. Les Cryptates. Tetrahedron Lett. 1969, 10, 2889–2892. 10.1016/S0040-4039(01)88300-3. - DOI
- Lehn J. M. Cryptates: Inclusion Complexes of Macropolycyclic Receptor Molecules. Pure Appl. Chem. 1978, 50, 871–892. 10.1351/pac197850090871. - DOI
- Lehn J. M. Supramolecular Chemistry - Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem., Int. Ed. Engl. 1988, 27, 89–112. 10.1002/anie.198800891. - DOI
-
-
-
For examples, see:
- Armstrong C. M. The Na/K Pump, Cl Ion, and Osmotic Stabilization of Cells. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 6257–6262. 10.1073/pnas.0931278100. - DOI - PMC - PubMed
- Gale P. A.; Busschaert N.; Haynes C. J. E.; Karagiannidis L. E.; Kirby I. L. Anion Receptor Chemistry: Highlights from 2011 and 2012. Chem. Soc. Rev. 2014, 43, 205–241. 10.1039/C3CS60316D. - DOI - PubMed
- Salis A.; Ninham B. W. Models and Mechanisms of Hofmeister Effects in Electrolyte Solutions, and Colloid and Protein Systems Revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. 10.1039/C4CS00144C. - DOI - PubMed
- Evans N. H.; Beer P. D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem., Int. Ed. 2014, 53, 11716–11754. 10.1002/anie.201309937. - DOI - PubMed
- Busschaert N.; Caltagirone C.; Van Rossom W.; Gale P. A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. 10.1021/acs.chemrev.5b00099. - DOI - PubMed
- Tapia L.; Pérez Y.; Bolte M.; Casas J.; Solà J.; Quesada R.; Alfonso I. pH-Dependent Chloride Transport by Pseudopeptidic Cages for the Selective Killing of Cancer Cells in Acidic Microenvironments. Angew. Chem., Int. Ed. 2019, 58, 12465–12468. 10.1002/anie.201905965. - DOI - PubMed
-
-
-
For examples, see:
- Mahadevi A. S.; Sastry G. N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. 10.1021/cr500344e. - DOI - PubMed
- Molina P.; Zapata F.; Caballero A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. 10.1021/acs.chemrev.6b00814. - DOI - PubMed
-
-
-
For examples, see:
- Dydio P.; Lichosyt D.; Jurczak J. Amide- and Urea-Functionalized Pyrroles and Benzopyrroles as Synthetic, Neutral Anion Receptors. Chem. Soc. Rev. 2011, 40, 2971–2985. 10.1039/c1cs15006e. - DOI - PubMed
- Jurczak J.; Dydio P.; Stępniak P.; Zieliński T. Diamidonaphthalenodipyrrole-Derived Fluorescent Sensors for Anions. Sens. Actuators, B 2016, 237, 621–627. 10.1016/j.snb.2016.06.142. - DOI
-
LinkOut - more resources
Full Text Sources

