The PCORnet Blood Pressure Control Laboratory: A Platform for Surveillance and Efficient Trials
- PMID: 32142371
- PMCID: PMC10681810
- DOI: 10.1161/CIRCOUTCOMES.119.006115
The PCORnet Blood Pressure Control Laboratory: A Platform for Surveillance and Efficient Trials
Abstract
Background: Uncontrolled blood pressure (BP) is a leading preventable cause of death that remains common in the US population despite the availability of effective medications. New technology and program innovation has high potential to improve BP but may be expensive and burdensome for patients, clinicians, health systems, and payers and may not produce desired results or reduce existing disparities in BP control.
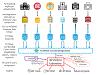
Methods and results: The PCORnet Blood Pressure Control Laboratory is a platform designed to enable national surveillance and facilitate quality improvement and comparative effectiveness research. The platform uses PCORnet, the National Patient-Centered Clinical Research Network, for engagement of health systems and collection of electronic health record data, and the Eureka Research Platform for eConsent and collection of patient-reported outcomes and mHealth data from wearable devices and smartphones. Three demonstration projects are underway: BP track will conduct national surveillance of BP control and related clinical processes by measuring theory-derived pragmatic BP control metrics using electronic health record data, with a focus on tracking disparities over time; BP MAP will conduct a cluster-randomized trial comparing effectiveness of 2 versions of a BP control quality improvement program; BP Home will conduct an individual patient-level randomized trial comparing effectiveness of smartphone-linked versus standard home BP monitoring. Thus far, BP Track has collected electronic health record data from over 826 000 eligible patients with hypertension who completed ≈3.1 million ambulatory visits. Preliminary results demonstrate substantial room for improvement in BP control (<140/90 mm Hg), which was 58% overall, and in the clinical processes relevant for BP control. For example, only 12% of patients with hypertension with a high BP measurement during an ambulatory visit received an order for a new antihypertensive medication.
Conclusions: The PCORnet Blood Pressure Control Laboratory is designed to be a reusable platform for efficient surveillance and comparative effectiveness research; results from demonstration projects are forthcoming.
Keywords: blood pressure; quality improvement; randomized controlled trial; smartphone.
Conflict of interest statement
DISCLOSURES
The authors have no other conflicts to report.
Figures


References
-
- Global Burden of Disease Risk Factors. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. - PMC - PubMed
-
- Institute for Health Metrics and Evaluation. GBD Compare. https://vizhub.healthdata.org/gbd-compare/. Accessed July 5, 2019.
-
- Nwankwo T, Yoon SS, Burt V and Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013:1–8. - PubMed
-
- Wald DS, Law M, Morris JK, Bestwick JP and Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. - PubMed

