GRP receptor and AMPA receptor cooperatively regulate itch-responsive neurons in the spinal dorsal horn
- PMID: 32142790
- PMCID: PMC7754563
- DOI: 10.1016/j.neuropharm.2020.108025
GRP receptor and AMPA receptor cooperatively regulate itch-responsive neurons in the spinal dorsal horn
Abstract
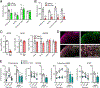
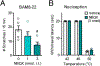
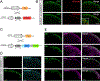
Gastrin-releasing peptide (GRP) receptor-expressing (GRPR)+ neurons have a central role in the spinal transmission of itch. Because their fundamental regulatory mechanisms are not yet understood, it is important to determine how such neurons are excited and integrate itch sensation. In this study, we investigated the mechanisms for the activation of itch-responsive GRPR+ neurons in the spinal dorsal horn (SDH). GRPR+ neurons expressed the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) containing the GluR2 subunit. In mice, peripherally elicited histaminergic and non-histaminergic itch was prevented by intrathecal (i.t.) administration of the AMPAR antagonist NBQX, which was consistent with the fact that firing of GRPR+ neurons in SDH under histaminergic and non-histaminergic itch was completely blocked by NBQX, but not by the GRPR antagonist RC-3095. Because GRP+ neurons in SDH contain glutamate, we investigated the role of GRP+ (GRP+/Glu+) neurons in regulating itch. Chemogenetic inhibition of GRP+ neurons suppressed both histaminergic and non-histaminergic itch without affecting the mechanical pain threshold. In nonhuman primates, i.t. administration of NBQX also attenuated peripherally elicited itch without affecting the thermal pain threshold. In a mouse model of diphenylcyclopropenone (DCP)-induced contact dermatitis, GRP, GRPR, and AMPAR subunits were upregulated in SDH. DCP-induced itch was prevented by either silencing GRP+ neurons or ablation of GRPR+ neurons. Altogether, these findings demonstrate that GRP and glutamate cooperatively regulate GRPR+ AMPAR+ neurons in SDH, mediating itch sensation. GRP-GRPR and the glutamate-AMPAR system may play pivotal roles in the spinal transmission of itch in rodents and nonhuman primates.
Keywords: Chloroquine; Glutamate; Histamine; Nonhuman primate; Pain; Pruritus.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures



Similar articles
-
Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch.Pain. 2014 Jan;155(1):80-92. doi: 10.1016/j.pain.2013.09.011. Epub 2013 Sep 13. Pain. 2014. PMID: 24041961 Free PMC article.
-
Critical role of GRP receptor-expressing neurons in the spinal transmission of imiquimod-induced psoriatic itch.Neuropsychopharmacol Rep. 2020 Sep;40(3):287-290. doi: 10.1002/npr2.12120. Epub 2020 Jun 25. Neuropsychopharmacol Rep. 2020. PMID: 32584520 Free PMC article.
-
Functional roles of neuromedin B and gastrin-releasing peptide in regulating itch and pain in the spinal cord of non-human primates.Biochem Pharmacol. 2022 Apr;198:114972. doi: 10.1016/j.bcp.2022.114972. Epub 2022 Feb 18. Biochem Pharmacol. 2022. PMID: 35189108 Free PMC article.
-
Itch signaling in the nervous system.Physiology (Bethesda). 2011 Aug;26(4):286-92. doi: 10.1152/physiol.00007.2011. Physiology (Bethesda). 2011. PMID: 21841076 Free PMC article. Review.
-
Recent insights into biological functions of mammalian bombesin-like peptides and their receptors.Curr Opin Endocrinol Diabetes Obes. 2018 Feb;25(1):36-41. doi: 10.1097/MED.0000000000000375. Curr Opin Endocrinol Diabetes Obes. 2018. PMID: 29120926 Review.
Cited by
-
Chemogenetic Activation of CX3CR1-Expressing Spinal Microglia Using Gq-DREADD Elicits Mechanical Allodynia in Male Mice.Cells. 2021 Apr 12;10(4):874. doi: 10.3390/cells10040874. Cells. 2021. PMID: 33921365 Free PMC article.
-
Chemogenetic Regulation of CX3CR1-Expressing Microglia Using Gi-DREADD Exerts Sex-Dependent Anti-Allodynic Effects in Mouse Models of Neuropathic Pain.Front Pharmacol. 2020 Jun 19;11:925. doi: 10.3389/fphar.2020.00925. eCollection 2020. Front Pharmacol. 2020. PMID: 32636748 Free PMC article.
-
Characterization of the expression of gastrin-releasing peptide and its receptor in the trigeminal and spinal somatosensory systems of Japanese macaque monkeys: Insight into humans.J Comp Neurol. 2022 Nov;530(16):2804-2819. doi: 10.1002/cne.25376. Epub 2022 Jun 10. J Comp Neurol. 2022. PMID: 35686563 Free PMC article.
-
Identification of potential biomarkers for abdominal pain in IBS patients by bioinformatics approach.BMC Gastroenterol. 2021 Feb 2;21(1):48. doi: 10.1186/s12876-021-01626-7. BMC Gastroenterol. 2021. PMID: 33530940 Free PMC article.
-
Critical Players and Therapeutic Targets in Chronic Itch.Int J Mol Sci. 2022 Sep 1;23(17):9935. doi: 10.3390/ijms23179935. Int J Mol Sci. 2022. PMID: 36077340 Free PMC article. Review.
References
-
- Anastasi A, Erspamer V, Bucci M, 1971. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 27, 166–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

