Enabling sublingual peptide immunization with molecular self-assemblies
- PMID: 32143059
- PMCID: PMC7171596
- DOI: 10.1016/j.biomaterials.2020.119903
Enabling sublingual peptide immunization with molecular self-assemblies
Abstract
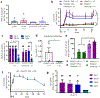
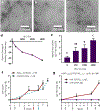
Short peptides are poorly immunogenic when delivered sublingually - under the tongue. Nanomaterial delivery of peptides could be utilized to improve immunogenicity towards designed sublingual vaccines, but nanomaterials have not been widely successful in sublingual vaccines owing to the challenges of transport through the sublingual mucosa. Here, we report that the sublingual immunogenicity of peptides is negligible, even in the presence of sublingual adjuvants or when PEGylated, but can be dramatically enhanced by assembly into supramolecular polymer-peptide nanofibers bearing low-molecular weight PEG, optimally between 2000 and 3000 Da. Neither PEGylation nor a sublingual adjuvant were capable of rendering peptides immunogenic without assembly into nanofibers. We found that PEG decreased nanofiber interactions with mucin and promoted longer residence time at the sublingual immunization site. Parallel investigations with shortened nanofibers indicated that the size of the assemblies had a surprisingly negligible influence over sublingual immunogenicity. In mice, optimized formulations were capable of raising strong and highly durable systemic antibody responses, antibodies in the upper respiratory and reproductive tracts, and systemic antigen-specific T-cell responses. These nanofiber-based sublingual vaccines were effective with both protein and nucleotide adjuvants and raised responses against both a model peptide epitope and a peptide epitope from M. tuberculosis. Further, PASylation (modification of nanofibers with peptide sequences rich in Pro, Ala, and Ser) could be substituted for PEGylation to also achieve sublingual immunogenicity. These findings indicated that surface properties supersede nanomaterial size in modulating sublingual nanomaterial immunogenicity, having important implications for the design of synthetic sublingual vaccines.
Keywords: Mucosal; Nanofiber; Self-assembly; Sublingual; Supramolecular; Vaccine.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JHC and SHK are listed as inventors on a patent application associated with the technology described.
Figures



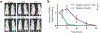
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

