Optimisation of cell and ex vivo culture conditions to study vascular calcification
- PMID: 32143215
- PMCID: PMC7060075
- DOI: 10.1371/journal.pone.0230201
Optimisation of cell and ex vivo culture conditions to study vascular calcification
Abstract
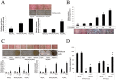
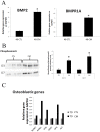
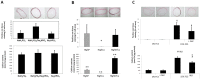
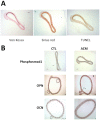
Medial vascular calcification (MVC) is a highly prevalent disease associated with a high risk of severe, potentially lethal, complications. While animal studies may not systematically be circumvented, in vitro systems have been proven useful to study disease physiopathology. In the context of MVC, the absence of a clinically relevant standardized in vitro method prevents the appropriate comparison and overall interpretation of results originating from different experiments. The aim of our study is to establish in vitro models mimicking in vivo vascular calcification and to select the best methods to unravel the mechanisms involved in MVC. Human aortic smooth muscle cells and rat aortic rings were cultured in different conditions. The influence of fetal calf serum (FCS), alkaline phosphatase, phosphate and calcium concentrations in the medium were evaluated. We identified culture conditions, including the herein reported Aorta Calcifying Medium (ACM), which allowed a reproducible and specific medial calcification of aortic explants. Studying cells and aortic explants cultured, the involvement of bone morphogenetic protein 2 (BMP2) pathway, fibrosis and apoptosis processes in in vitro MVC were demonstrated. Expression of osteoblastic markers was also observed suggesting the occurrence of transdifferentiation of smooth muscle cells to osteoblasts in our models. The use of these models will help researchers in the field of vascular calcification to achieve reproducible results and allow result comparison in a more consistent way.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures