Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice
- PMID: 32143275
- PMCID: PMC7084583
- DOI: 10.3390/ijms21051741
Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice
Erratum in
-
Correction: Cutuli et al. Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice. Int. J. Mol. Sci. 2020, 21, 1741.Int J Mol Sci. 2022 Jun 22;23(13):6916. doi: 10.3390/ijms23136916. Int J Mol Sci. 2022. PMID: 35806494 Free PMC article.
Abstract
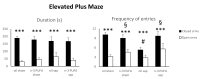
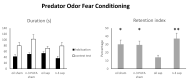
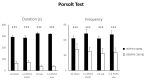
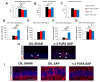
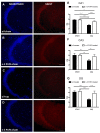
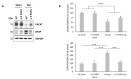
As major components of neuronal membranes, omega-3 polyunsaturated fatty acids (n-3 PUFA) exhibit a wide range of regulatory functions. Recent human and animal studies indicate that n-3 PUFA may exert beneficial effects on aging processes. Here we analyzed the neuroprotective influence of n-3 PUFA supplementation on behavioral deficits, hippocampal neurogenesis, volume loss, and astrogliosis in aged mice that underwent a selective depletion of basal forebrain cholinergic neurons. Such a lesion represents a valid model to mimic a key component of the cognitive deficits associated with dementia. Aged mice were supplemented with n-3 PUFA or olive oil (as isocaloric control) for 8 weeks and then cholinergically depleted with mu-p75-saporin immunotoxin. Two weeks after lesioning, mice were behaviorally tested to assess anxious, motivational, social, mnesic, and depressive-like behaviors. Subsequently, morphological and biochemical analyses were performed. In lesioned aged mice the n-3 PUFA pre-treatment preserved explorative skills and associative retention memory, enhanced neurogenesis in the dentate gyrus, and reduced volume and VAChT levels loss as well as astrogliosis in hippocampus. The present findings demonstrating that n-3 PUFA supplementation before cholinergic depletion can counteract behavioral deficits and hippocampal neurodegeneration in aged mice advance a low-cost, non-invasive preventive tool to enhance life quality during aging.
Keywords: aging; cholinergic system; cognitive deficits; neuroprotection; omega-3 fatty acids; prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Behavioral, neuromorphological, and neurobiochemical effects induced by omega-3 fatty acids following basal forebrain cholinergic depletion in aged mice.Alzheimers Res Ther. 2020 Nov 16;12(1):150. doi: 10.1186/s13195-020-00705-3. Alzheimers Res Ther. 2020. PMID: 33198763 Free PMC article.
-
Impaired hippocampal acetylcholine release parallels spatial memory deficits in Tg2576 mice subjected to basal forebrain cholinergic degeneration.Brain Res. 2014 Jan 16;1543:253-62. doi: 10.1016/j.brainres.2013.10.055. Epub 2013 Nov 11. Brain Res. 2014. PMID: 24231553
-
n-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice.Front Aging Neurosci. 2014 Aug 25;6:220. doi: 10.3389/fnagi.2014.00220. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25202271 Free PMC article.
-
Immunolesion by 192IgG-saporin of rat basal forebrain cholinergic system: a useful tool to produce cortical cholinergic dysfunction.Prog Brain Res. 1996;109:253-64. doi: 10.1016/s0079-6123(08)62109-3. Prog Brain Res. 1996. PMID: 9009714 Review.
-
Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms.Ageing Res Rev. 2013 Mar;12(2):579-94. doi: 10.1016/j.arr.2013.01.007. Epub 2013 Feb 6. Ageing Res Rev. 2013. PMID: 23395782 Review.
Cited by
-
Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect.Mar Drugs. 2022 Oct 25;20(11):662. doi: 10.3390/md20110662. Mar Drugs. 2022. PMID: 36354985 Free PMC article. Review.
-
A Healthy Gut for a Healthy Brain: Preclinical, Clinical and Regulatory Aspects.Curr Neuropharmacol. 2021;19(5):610-628. doi: 10.2174/1570159X18666200730111528. Curr Neuropharmacol. 2021. PMID: 32744976 Free PMC article. Review.
-
Mediterranean Diet on Sleep: A Health Alliance.Nutrients. 2022 Jul 21;14(14):2998. doi: 10.3390/nu14142998. Nutrients. 2022. PMID: 35889954 Free PMC article. Review.
-
Behavioral, neuromorphological, and neurobiochemical effects induced by omega-3 fatty acids following basal forebrain cholinergic depletion in aged mice.Alzheimers Res Ther. 2020 Nov 16;12(1):150. doi: 10.1186/s13195-020-00705-3. Alzheimers Res Ther. 2020. PMID: 33198763 Free PMC article.
-
Correction: Cutuli et al. Neuroprotective Role of Dietary Supplementation with Omega-3 Fatty Acids in the Presence of Basal Forebrain Cholinergic Neurons Degeneration in Aged Mice. Int. J. Mol. Sci. 2020, 21, 1741.Int J Mol Sci. 2022 Jun 22;23(13):6916. doi: 10.3390/ijms23136916. Int J Mol Sci. 2022. PMID: 35806494 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

