Development and Differentiation in Monobodies Based on the Fibronectin Type 3 Domain
- PMID: 32143310
- PMCID: PMC7140400
- DOI: 10.3390/cells9030610
Development and Differentiation in Monobodies Based on the Fibronectin Type 3 Domain
Abstract
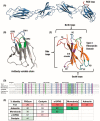
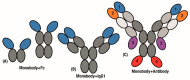
As a non-antibody scaffold, monobodies based on the fibronectin type III (FN3) domain overcome antibody size and complexity while maintaining analogous binding loops. However, antibodies and their derivatives remain the gold standard for the design of new therapeutics. In response, clinical-stage therapeutic proteins based on the FN3 domain are beginning to use native fibronectin function as a point of differentiation. The small and simple structure of monomeric monobodies confers increased tissue distribution and reduced half-life, whilst the absence of disulphide bonds improves stability in cytosolic environments. Where multi-specificity is challenging with an antibody format that is prone to mis-pairing between chains, multiple FN3 domains in the fibronectin assembly already interact with a large number of molecules. As such, multiple monobodies engineered for interaction with therapeutic targets are being combined in a similar beads-on-a-string assembly which improves both efficacy and pharmacokinetics. Furthermore, full length fibronectin is able to fold into multiple conformations as part of its natural function and a greater understanding of how mechanical forces allow for the transition between states will lead to advanced applications that truly differentiate the FN3 domain as a therapeutic scaffold.
Keywords: adnectin; biosensor; fibronectin; monobody; non-antibody scaffold; therapeutic.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


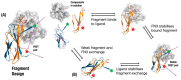
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

