Immunotherapy, Inflammation and Colorectal Cancer
- PMID: 32143413
- PMCID: PMC7140520
- DOI: 10.3390/cells9030618
Immunotherapy, Inflammation and Colorectal Cancer
Abstract
Colorectal cancer (CRC) is the third most common cancer type, and third highest in mortality rates among cancer-related deaths in the United States. Originating from intestinal epithelial cells in the colon and rectum, that are impacted by numerous factors including genetics, environment and chronic, lingering inflammation, CRC can be a problematic malignancy to treat when detected at advanced stages. Chemotherapeutic agents serve as the historical first line of defense in the treatment of metastatic CRC. In recent years, however, combinational treatment with targeted therapies, such as vascular endothelial growth factor, or epidermal growth factor receptor inhibitors, has proven to be quite effective in patients with specific CRC subtypes. While scientific and clinical advances have uncovered promising new treatment options, the five-year survival rate for metastatic CRC is still low at about 14%. Current research into the efficacy of immunotherapy, particularly immune checkpoint inhibitor therapy (ICI) in mismatch repair deficient and microsatellite instability high (dMMR-MSI-H) CRC tumors have shown promising results, but its use in other CRC subtypes has been either unsuccessful, or not extensively explored. This Review will focus on the current status of immunotherapies, including ICI, vaccination and adoptive T cell therapy (ATC) in the treatment of CRC and its potential use, not only in dMMR-MSI-H CRC, but also in mismatch repair proficient and microsatellite instability low (pMMR-MSI-L).
Keywords: colorectal cancer; immunotherapy; inflammation; microsatellite instability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

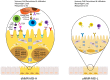
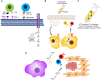
References
-
- American Cancer Society Cancer Facts & Figures, American Cancer Society, Atlanta, Georgia, 2019. [(accessed on 4 January 2020)]; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-....
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

