A Novel Way of Preventing Postoperative Pancreatic Fistula by Directly Injecting Profibrogenic Materials into the Pancreatic Parenchyma
- PMID: 32143463
- PMCID: PMC7084673
- DOI: 10.3390/ijms21051759
A Novel Way of Preventing Postoperative Pancreatic Fistula by Directly Injecting Profibrogenic Materials into the Pancreatic Parenchyma
Abstract
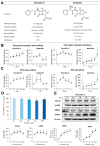
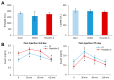
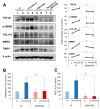
This paper aims to validate if intrapancreatic injection of penicillin G can enhance hardness and suture holding capacity (SHC) of the pancreas through prompting the fibrosis process. Soft pancreatic texture is constantly mentioned as one of the most contributory predictors of postoperative pancreatic fistula (POPF). Soft pancreas has poor SHC and higher incidence of parenchymal tearing, frequently leading to POPF. From a library of 114 antibiotic compounds, we identified that penicillin G substantially enhanced pancreatic hardness and SHC in experimental mice. Specifically, we injected penicillin G directly into the pancreas. On determined dates, we measured the pancreatic hardness and SHC, respectively, and performed molecular and histological examinations for estimation of the degree of fibrosis. The intrapancreatic injection of penicillin G activated human pancreatic stellate cells (HPSCs) to produce various fibrotic materials such as transforming growth factor-β1 (TGF-β1) and metalloproteinases-2. The pancreatic hardness and SHC were increased to the maximum at the second day after injection and then it gradually subsided demonstrating its reversibility. Pretreatment of mice with SB431542, an inhibitor of the TGF-β1 receptor, before injecting penicillin G intrapancreatically, significantly abrogated the increase of both pancreatic hardness and SHC caused by penicillin G. This suggested that penicillin G promotes pancreatic fibrosis through the TGF-β1 signaling pathway. Intrapancreatic injection of penicillin G promotes pancreatic hardness and SHC by enhancing pancreatic fibrosis. We thus think that penicillin G could be utilized to prevent and minimize POPF, after validating its actual effectiveness and safety by further studies.
Keywords: fibrosis; pancreas texture; pancreatic stellate cells; penicillin G; postoperative pancreatic fistula (POPF); transforming growth factor-β1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

