Designs for adding a treatment arm to an ongoing clinical trial
- PMID: 32143729
- PMCID: PMC7060622
- DOI: 10.1186/s13063-020-4073-1
Designs for adding a treatment arm to an ongoing clinical trial
Abstract
Background: For many disease areas, there are often treatments in different stages of the development process. We consider the design of a two-arm parallel group trial where it is planned to add a new experimental treatment arm during the trial. This could potentially save money, patients, time and resources; however, the addition of a treatment arm creates a multiple comparison problem. Current practice in trials when a new treatment arm has been added is to compare the new treatment only to controls randomised concurrently, and this is the setting we consider here. Furthermore, for standard multi-arm trials, optimal allocation randomises a larger number of patients to the control arm than to each experimental treatment arm.
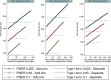
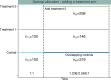
Methods: In this paper we propose an adaptive design, the aim of which is to adapt the sample size of the trial when the new treatment arm is added to control the family-wise error rate (FWER) in the strong sense, whilst maintaining the marginal power of each treatment-to-control comparison at the level of the original study. We explore optimal allocation for designs where a treatment arm is added with the aim of increasing the overall power of the study, where we define the overall power to be the probability of detecting all treatments that are better than the control.
Results and conclusions: An increase in sample size is required to maintain the marginal power for each pairwise comparison when adding a treatment arm if control of the FWER is required at the level of the type I error in the original study. When control of the FWER is required in a single trial which adds an additional experimental treatment arm, but control of the FWER is not required in separate trials, depending on the design characteristics, it may be better to run a separate trial for each experimental treatment, in terms of the number of patients required. An increase in overall power can be achieved when optimal allocation is used once a treatment arm has been added to the trial, rather than continuing with equal allocation to all treatment arms.
Keywords: Adaptive design; Adding a treatment arm; Family-wise error rate control; Multiple testing; Optimal allocation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50(272):1096–121. doi: 10.1080/01621459.1955.10501294. - DOI
-
- StataCorp. Stata Statistical Software: Release 13. 2013. https://www.stata.com/stata13/. Accessed June 2013.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

