Genetic Influences of the Microbiota on the Life Span of Drosophila melanogaster
- PMID: 32144104
- PMCID: PMC7205492
- DOI: 10.1128/AEM.00305-20
Genetic Influences of the Microbiota on the Life Span of Drosophila melanogaster
Abstract
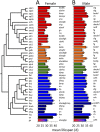
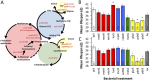
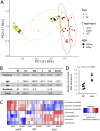
To better understand how associated microorganisms ("microbiota") influence organismal aging, we focused on the model organism Drosophila melanogaster We conducted a metagenome-wide association (MGWA) as a screen to identify bacterial genes associated with variation in the D. melanogaster life span. The results of the MGWA predicted that bacterial cysteine and methionine metabolism genes influence fruit fly longevity. A mutant analysis, in which flies were inoculated with Escherichia coli strains bearing mutations in various methionine cycle genes, confirmed a role for some methionine cycle genes in extending or shortening fruit fly life span. Initially, we predicted these genes might influence longevity by mimicking or opposing methionine restriction, an established mechanism for life span extension in fruit flies. However, follow-up transcriptome sequencing (RNA-seq) and metabolomic experiments were generally inconsistent with this conclusion and instead implicated glucose and vitamin B6 metabolism in these influences. We then tested if bacteria could influence life span through methionine restriction using a different set of bacterial strains. Flies reared with a bacterial strain that ectopically expressed bacterial transsulfuration genes and lowered the methionine content of the fly diet also extended female D. melanogaster life span. Taken together, the microbial influences shown here overlap with established host genetic mechanisms for aging and therefore suggest overlapping roles for host and microbial metabolism genes in organismal aging.IMPORTANCE Associated microorganisms ("microbiota") are intimately connected to the behavior and physiology of their animal hosts, and defining the mechanisms of these interactions is an urgent imperative. This study focuses on how microorganisms influence the life span of a model host, the fruit fly Drosophila melanogaster First, we performed a screen that suggested a strong influence of bacterial methionine metabolism on host life span. Follow-up analyses of gene expression and metabolite abundance identified stronger roles for vitamin B6 and glucose than methionine metabolism among the tested mutants, possibly suggesting a more limited role for bacterial methionine metabolism genes in host life span effects. In a parallel set of experiments, we created a distinct bacterial strain that expressed life span-extending methionine metabolism genes and showed that this strain can extend fly life span. Therefore, this work identifies specific bacterial genes that influence host life span, including in ways that are consistent with the expectations of methionine restriction.
Keywords: Acetobacter; Drosophila melanogaster; Lactobacillus; MGWA; glucose; life span; metagenome-wide association; methionine restriction; microbiota; vitamin B6.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Khanna A, Kumar J, Vargas MA, Barrett L, Katewa S, Li P, McCloskey T, Sharma A, Naude N, Nelson C, Brem R, Killilea DW, Mooney SD, Gill M, Kapahi P. 2016. A genome-wide screen of bacterial mutants that enhance dauer formation in C. elegans. Sci Rep 6:38764. doi:10.1038/srep38764. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

