Substrate recognition and ATPase activity of the E. coli cysteine/cystine ABC transporter YecSC-FliY
- PMID: 32144203
- PMCID: PMC7170509
- DOI: 10.1074/jbc.RA119.012063
Substrate recognition and ATPase activity of the E. coli cysteine/cystine ABC transporter YecSC-FliY
Abstract
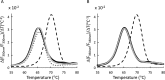
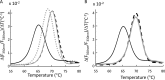
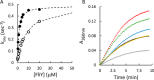
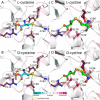
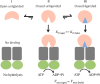
Sulfur is essential for biological processes such as amino acid biogenesis, iron-sulfur cluster formation, and redox homeostasis. To acquire sulfur-containing compounds from the environment, bacteria have evolved high-affinity uptake systems, predominant among which is the ABC transporter family. Theses membrane-embedded enzymes use the energy of ATP hydrolysis for transmembrane transport of a wide range of biomolecules against concentration gradients. Three distinct bacterial ABC import systems of sulfur-containing compounds have been identified, but the molecular details of their transport mechanism remain poorly characterized. Here we provide results from a biochemical analysis of the purified Escherichia coli YecSC-FliY cysteine/cystine import system. We found that the substrate-binding protein FliY binds l-cystine, l-cysteine, and d-cysteine with micromolar affinities. However, binding of the l- and d-enantiomers induced different conformational changes of FliY, where the l- enantiomer-substrate-binding protein complex interacted more efficiently with the YecSC transporter. YecSC had low basal ATPase activity that was moderately stimulated by apo FliY, more strongly by d-cysteine-bound FliY, and maximally by l-cysteine- or l-cystine-bound FliY. However, at high FliY concentrations, YecSC reached maximal ATPase rates independent of the presence or nature of the substrate. These results suggest that FliY exists in a conformational equilibrium between an open, unliganded form that does not bind to the YecSC transporter and closed, unliganded and closed, liganded forms that bind this transporter with variable affinities but equally stimulate its ATPase activity. These findings differ from previous observations for similar ABC transporters, highlighting the extent of mechanistic diversity in this large protein family.
Keywords: ABC transporter; ATPase; YecSC-FliY; amino acid transport; cysteine/cystine import; enantiomers; enzyme mechanism; membrane protein; membrane transport; sulfur.
© 2020 Sabrialabed et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Holland I. B., Cole S. P. C., Kuchler K., and Higgins C. F. (2003) ABC Proteins: From Bacteria to Man, Academic Press, San Diego, CA
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

