Glucose transporters in the kidney in health and disease
- PMID: 32144488
- PMCID: PMC7483786
- DOI: 10.1007/s00424-020-02361-w
Glucose transporters in the kidney in health and disease
Abstract
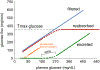
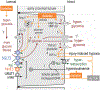
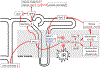
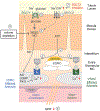
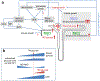
The kidneys filter large amounts of glucose. To prevent the loss of this valuable fuel, the tubular system of the kidney, particularly the proximal tubule, has been programmed to reabsorb all filtered glucose. The machinery involves the sodium-glucose cotransporters SGLT2 and SGLT1 on the apical membrane and the facilitative glucose transporter GLUT2 on the basolateral membrane. The proximal tubule also generates new glucose, particularly in the post-absorptive phase but also to enhance bicarbonate formation and maintain acid-base balance. The glucose reabsorbed or formed by the proximal tubule is primarily taken up into peritubular capillaries and returned to the systemic circulation or provided as an energy source to further distal tubular segments that take up glucose by basolateral GLUT1. Recent studies provided insights on the coordination of renal glucose reabsorption, formation, and usage. Moreover, a better understanding of renal glucose transport in disease states is emerging. This includes the kidney in diabetes mellitus, when renal glucose retention becomes maladaptive and contributes to hyperglycemia. Furthermore, enhanced glucose reabsorption is coupled to sodium retention through the sodium-glucose cotransporter SGLT2, which induces secondary deleterious effects. As a consequence, SGLT2 inhibitors are new anti-hyperglycemic drugs that can protect the kidneys and heart from failing. Recent studies discovered unique roles for SGLT1 with implications in acute kidney injury and glucose sensing at the macula densa. This review discusses established and emerging concepts of renal glucose transport, and outlines the need for a better understanding of renal glucose handling in health and disease.
Keywords: Diabetic nephropathy; GLUT1; Gluconeogenesis; Glucose transport; SGLT1; SGLT2 inhibition.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

