Reversal of Social Recognition Deficit in Adult Mice with MECP2 Duplication via Normalization of MeCP2 in the Medial Prefrontal Cortex
- PMID: 32144612
- PMCID: PMC7271088
- DOI: 10.1007/s12264-020-00467-w
Reversal of Social Recognition Deficit in Adult Mice with MECP2 Duplication via Normalization of MeCP2 in the Medial Prefrontal Cortex
Abstract
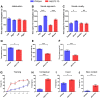
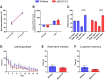
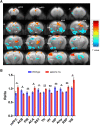
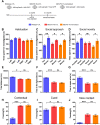
Methyl-CpG binding protein 2 (MeCP2) is a basic nuclear protein involved in the regulation of gene expression and microRNA processing. Duplication of MECP2-containing genomic segments causes MECP2 duplication syndrome, a severe neurodevelopmental disorder characterized by intellectual disability, motor dysfunction, heightened anxiety, epilepsy, autistic phenotypes, and early death. Reversal of the abnormal phenotypes in adult mice with MECP2 duplication (MECP2-TG) by normalizing the MeCP2 levels across the whole brain has been demonstrated. However, whether different brain areas or neural circuits contribute to different aspects of the behavioral deficits is still unknown. Here, we found that MECP2-TG mice showed a significant social recognition deficit, and were prone to display aversive-like behaviors, including heightened anxiety-like behaviors and a fear generalization phenotype. In addition, reduced locomotor activity was observed in MECP2-TG mice. However, appetitive behaviors and learning and memory were comparable in MECP2-TG and wild-type mice. Functional magnetic resonance imaging illustrated that the differences between MECP2-TG and wild-type mice were mainly concentrated in brain areas regulating emotion and social behaviors. We used the CRISPR-Cas9 method to restore normal MeCP2 levels in the medial prefrontal cortex (mPFC) and bed nuclei of the stria terminalis (BST) of adult MECP2-TG mice, and found that normalization of MeCP2 levels in the mPFC but not in the BST reversed the social recognition deficit. These data indicate that the mPFC is responsible for the social recognition deficit in the transgenic mice, and provide new insight into potential therapies for MECP2 duplication syndrome.
Keywords: CRISPR-Cas9; MECP2 duplication; Medial prefrontal cortex; Social recognition deficit.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Comment in
-
Gene Editing to the Rescue: Reversal of Social Deficits Associated with MECP2 Duplication.Neurosci Bull. 2020 Jun;36(6):567-569. doi: 10.1007/s12264-020-00522-6. Epub 2020 May 29. Neurosci Bull. 2020. PMID: 32472288 Free PMC article. No abstract available.
Similar articles
-
Human MECP2 transgenic rats show increased anxiety, severe social deficits, and abnormal prefrontal neural oscillation stability.Biochem Biophys Res Commun. 2023 Mar 12;648:28-35. doi: 10.1016/j.bbrc.2023.01.057. Epub 2023 Jan 20. Biochem Biophys Res Commun. 2023. PMID: 36724557
-
Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides.Nature. 2015 Dec 3;528(7580):123-6. doi: 10.1038/nature16159. Epub 2015 Nov 25. Nature. 2015. PMID: 26605526 Free PMC article.
-
Activation of the Medial Prefrontal Cortex Reverses Cognitive and Respiratory Symptoms in a Mouse Model of Rett Syndrome.eNeuro. 2018 Jan 10;4(6):ENEURO.0277-17.2017. doi: 10.1523/ENEURO.0277-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2018. PMID: 29333487 Free PMC article.
-
Deciphering MECP2-associated disorders: disrupted circuits and the hope for repair.Curr Opin Neurobiol. 2018 Feb;48:30-36. doi: 10.1016/j.conb.2017.09.004. Epub 2017 Sep 27. Curr Opin Neurobiol. 2018. PMID: 28961504 Review.
-
[Advance in research on MECP2 [corrected] duplication syndrome].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2015 Jun;32(3):426-9. doi: 10.3760/cma.j.issn.1003-9406.2015.03.028. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2015. PMID: 26037367 Review. Chinese.
Cited by
-
Shank2/3 double knockout-based screening of cortical subregions links the retrosplenial area to the loss of social memory in autism spectrum disorders.Mol Psychiatry. 2022 Dec;27(12):4994-5006. doi: 10.1038/s41380-022-01756-8. Epub 2022 Sep 13. Mol Psychiatry. 2022. PMID: 36100669 Free PMC article.
-
Overexpression of MECP2 in the Suprachiasmatic Nucleus Alters Circadian Rhythm and Induces Abnormal Social Behaviors.Neurosci Bull. 2021 Dec;37(12):1713-1717. doi: 10.1007/s12264-021-00746-0. Epub 2021 Jul 20. Neurosci Bull. 2021. PMID: 34283398 Free PMC article. No abstract available.
-
An RNA editing strategy rescues gene duplication in a mouse model of MECP2 duplication syndrome and nonhuman primates.Nat Neurosci. 2025 Jan;28(1):72-83. doi: 10.1038/s41593-024-01838-6. Epub 2024 Dec 12. Nat Neurosci. 2025. PMID: 39668251
-
Development of prefrontal cortex.Neuropsychopharmacology. 2022 Jan;47(1):41-57. doi: 10.1038/s41386-021-01137-9. Epub 2021 Oct 13. Neuropsychopharmacology. 2022. PMID: 34645980 Free PMC article. Review.
-
Chromatin Alterations in Neurological Disorders and Strategies of (Epi)Genome Rescue.Pharmaceuticals (Basel). 2021 Aug 4;14(8):765. doi: 10.3390/ph14080765. Pharmaceuticals (Basel). 2021. PMID: 34451862 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

