Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic
- PMID: 32144830
- PMCID: PMC8247289
- DOI: 10.1002/anie.202000451
Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic
Abstract
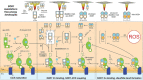
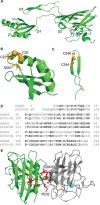
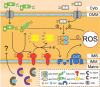
Cu/Zn superoxide dismutase (SOD1) is a frontline antioxidant enzyme catalysing superoxide breakdown and is important for most forms of eukaryotic life. The evolution of aerobic respiration by mitochondria increased cellular production of superoxide, resulting in an increased reliance upon SOD1. Consistent with the importance of SOD1 for cellular health, many human diseases of the central nervous system involve perturbations in SOD1 biology. But far from providing a simple demonstration of how disease arises from SOD1 loss-of-function, attempts to elucidate pathways by which atypical SOD1 biology leads to neurodegeneration have revealed unexpectedly complex molecular characteristics delineating healthy, functional SOD1 protein from that which likely contributes to central nervous system disease. This review summarises current understanding of SOD1 biology from SOD1 genetics through to protein function and stability.
Keywords: Cu/Zn superoxide dismutase; antioxidants; copper; neurodegeneration; protein misfolding.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


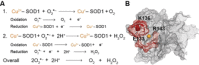



References
-
- Karnati S., Luers G., Pfreimer S., Baumgart-Vogt E., Histochem. Cell Biol. 2013, 140, 105–117. - PubMed
-
- Oury T. D., Day B. J., Crapo J. D., Free Radical Biol. Med. 1996, 20, 957–965. - PubMed
-
- Mann T., Kleinin D., Proc. R. Soc. London Ser. B 1938, 126, 303–315.
-
- McCord J. M., Fridovic I., J. Biol. Chem. 1969, 244, 6049. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

