Predicting Confined 1D Cell Migration from Parameters Calibrated to a 2D Motor-Clutch Model
- PMID: 32145191
- PMCID: PMC7136340
- DOI: 10.1016/j.bpj.2020.01.048
Predicting Confined 1D Cell Migration from Parameters Calibrated to a 2D Motor-Clutch Model
Abstract
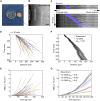
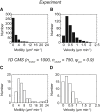
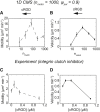
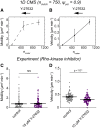
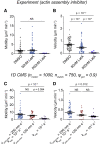
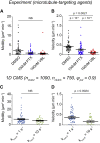
Biological tissues contain micrometer-scale gaps and pores, including those found within extracellular matrix fiber networks, between tightly packed cells, and between blood vessels or nerve bundles and their associated basement membranes. These spaces restrict cell motion to a single-spatial dimension (1D), a feature that is not captured in traditional in vitro cell migration assays performed on flat, unconfined two-dimensional (2D) substrates. Mechanical confinement can variably influence cell migration behaviors, and it is presently unclear whether the mechanisms used for migration in 2D unconfined environments are relevant in 1D confined environments. Here, we assessed whether a cell migration simulator and associated parameters previously measured for cells on 2D unconfined compliant hydrogels could predict 1D confined cell migration in microfluidic channels. We manufactured microfluidic devices with narrow channels (60-μm2 rectangular cross-sectional area) and tracked human glioma cells that spontaneously migrated within channels. Cell velocities (vexp = 0.51 ± 0.02 μm min-1) were comparable to brain tumor expansion rates measured in the clinic. Using motor-clutch model parameters estimated from cells on unconfined 2D planar hydrogel substrates, simulations predicted similar migration velocities (vsim = 0.37 ± 0.04 μm min-1) and also predicted the effects of drugs targeting the motor-clutch system or cytoskeletal assembly. These results are consistent with glioma cells utilizing a motor-clutch system to migrate in confined environments.
Copyright © 2020 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Lefranc F., Brotchi J., Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J. Clin. Oncol. 2005;23:2411–2422. - PubMed
-
- Lauffenburger D.A., Horwitz A.F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. - PubMed
-
- Prahl L.S., Odde D.J. Modeling cell migration mechanics. Adv. Exp. Med. Biol. 2018;1092:159–187. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

