Gemcitabine Recruits M2-Type Tumor-Associated Macrophages into the Stroma of Pancreatic Cancer
- PMID: 32145636
- PMCID: PMC7058407
- DOI: 10.1016/j.tranon.2020.01.004
Gemcitabine Recruits M2-Type Tumor-Associated Macrophages into the Stroma of Pancreatic Cancer
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is a very lethal disease that can develop therapy resistance over time. The dense stroma in PDAC plays a critical role in tumor progression and resistance. How this stroma interacts with the tumor cells and how this is influenced by chemotherapy remain poorly understood.
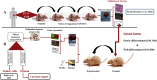
Methods: The backbone of this study is the parallel transcriptome analysis of human tumor and mouse stroma in two molecular and clinical representative patient-derived tumor xenografts models. Mice (8 animals per group) were treated for 4 weeks with gemcitabine or control. We studied tumor growth, RNA expression in the stroma, tumor-associated macrophages (TAMs) with immunofluorescence, and cytokines in the serum.
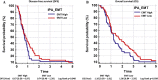
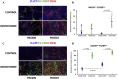
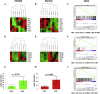
Results: A method for parallel transcriptome analysis was optimized. We found that the tumor (differentiation, gene expression) determines the infiltration of macrophages into the stroma. In aggressive PDAC (epithelial-to-mesenchymal transition high), we find more M2 polarized TAMs and the activation of cytokines and growth factors (TNFα, TGFβ1, and IL6). There are increased stromal glycolysis, reduced fatty acid oxidation, and reduced mitochondrial oxidation (tricarboxylic acid cycle and oxidative phosphorylation). Treatment with gemcitabine results in a shift of innate immune cells, especially additional infiltration of protumoral M2 TAMs (P < .001) and metabolic reprogramming.
Conclusions: Gemcitabine treatment of PDAC xenografts stimulates a protumoral macrophage phenotype, and this, in combination with a shift of the tumor cells to a mesenchymal phenotype that we reported previously, contributes to tumor progression and therapeutic resistance. Targeting M2-polarized TAMs may benefit PDAC patients at risk to become refractory to current anticancer regimens.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Luo G, Long J, Zhang B, Liu C, Xu J, Ni Q, Yu X. Stroma and pancreatic ductal adenocarcinoma: an interaction loop. Biochimica Biophysica Acta. 2012;1826:170–178. - PubMed
-
- Kota J, Hancock J, Kwon J, Korc M. Pancreatic cancer: stroma and its current and emerging targeted therapies. Cancer Lett. 2017;391:38–49. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

