Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling
- PMID: 32146229
- PMCID: PMC7610230
- DOI: 10.1016/j.neuropharm.2020.108021
Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling
Abstract
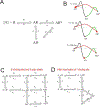
Postsynaptic nAChRs in the peripheral nervous system are critical for neuromuscular and autonomic neurotransmission. Pre- and peri-synaptic nAChRs in the brain modulate neurotransmission and are responsible for the addictive effects of nicotine. Subtypes of nAChRs in lymphocytes and non-synaptic locations may modulate inflammation and other cellular functions. All AChRs that function as ligand-gated ion channels are formed from five homologous subunits organized to form a central cation channel whose opening is regulated by ACh bound at extracellular subunit interfaces. nAChR subtype subunit composition can range from α7 homomers to α4β2α6β2β3 heteromers. Subtypes differ in affinities for ACh and other agonists like nicotine and in efficiencies with which their channels are opened and desensitized. Subtypes also differ in affinities for antagonists and for positive and negative allosteric modulators. Some agonists are "silent" with respect to channel opening, and AChRs may be able to signal metabotropic pathways by releasing G-proteins independent of channel opening. Electrophysiological studies that can resolve single-channel openings and molecular genetic approaches have allowed characterization of the structures of ligand binding sites, the cation channel, and the linkages between them, as well as the organization of AChR subunits and their contributions to function. Crystallography and cryo-electron-microscopy are providing increasing insights into the structures and functions of AChRs. However, much remains to be learned about both AChR structure and function, the in vivo functional roles of some AChR subtypes, and the development of better pharmacological tools directed at AChRs to treat addiction, pain, inflammation, and other medically important issues. This article is part of the special issue on 'Contemporary Advances in Nicotine Neuropharmacology'.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
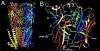



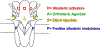


Similar articles
-
Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors.Neurochem Int. 2000 Jun;36(7):595-645. doi: 10.1016/s0197-0186(99)00154-0. Neurochem Int. 2000. PMID: 10771117 Review.
-
Lessons from nature: Structural studies and drug design driven by a homologous surrogate from invertebrates, AChBP.Neuropharmacology. 2020 Nov 15;179:108108. doi: 10.1016/j.neuropharm.2020.108108. Epub 2020 Apr 27. Neuropharmacology. 2020. PMID: 32353365 Review.
-
Differential pharmacological activity of JN403 between α7 and muscle nicotinic acetylcholine receptors.Biochemistry. 2013 Nov 26;52(47):8480-8. doi: 10.1021/bi4012572. Epub 2013 Nov 11. Biochemistry. 2013. PMID: 24164482
-
Advances in the In vitro and In vivo pharmacology of Alpha4beta2 nicotinic receptor positive allosteric modulators.Neuropharmacology. 2020 May 15;168:108008. doi: 10.1016/j.neuropharm.2020.108008. Epub 2020 Feb 12. Neuropharmacology. 2020. PMID: 32113032 Free PMC article. Review.
-
Allosteric modulation of nicotinic acetylcholine receptors.Biochem Pharmacol. 2015 Oct 15;97(4):408-417. doi: 10.1016/j.bcp.2015.07.028. Epub 2015 Jul 29. Biochem Pharmacol. 2015. PMID: 26231943 Review.
Cited by
-
Novel Three-Finger Neurotoxins from Naja melanoleuca Cobra Venom Interact with GABAA and Nicotinic Acetylcholine Receptors.Toxins (Basel). 2021 Feb 20;13(2):164. doi: 10.3390/toxins13020164. Toxins (Basel). 2021. PMID: 33672715 Free PMC article.
-
Meta-Analysis on Nicotine's Modulation of HIV-Associated Dementia.J Neuroimmune Pharmacol. 2022 Dec;17(3-4):487-502. doi: 10.1007/s11481-021-10027-2. Epub 2021 Nov 10. J Neuroimmune Pharmacol. 2022. PMID: 34757527 Free PMC article.
-
Cannabinoids, Inner Ear, Hearing, and Tinnitus: A Neuroimmunological Perspective.Front Neurol. 2020 Nov 23;11:505995. doi: 10.3389/fneur.2020.505995. eCollection 2020. Front Neurol. 2020. PMID: 33329293 Free PMC article. Review.
-
The Role of Alpha-7 Nicotinic Acetylcholine Receptors in Pain: Potential Therapeutic Implications.Curr Neuropharmacol. 2025;23(2):129-144. doi: 10.2174/1570159X22666240528161117. Curr Neuropharmacol. 2025. PMID: 38808717 Free PMC article. Review.
-
A Functional Interaction Between Y674-R685 Region of the SARS-CoV-2 Spike Protein and the Human α7 Nicotinic Receptor.Mol Neurobiol. 2022 Oct;59(10):6076-6090. doi: 10.1007/s12035-022-02947-8. Epub 2022 Jul 20. Mol Neurobiol. 2022. PMID: 35859025 Free PMC article.
References
-
- Alkondon M, Albuquerque EX, 1993. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 265, 1455–1473. - PubMed
-
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX, 1997. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9, 2734–2742. - PubMed
-
- Anand R, Lindstrom J, 1992. Chromosomal localization of seven neuronal nicotinic acetylcholine receptor subunit genes in humans. Genomics 13, 962–967. - PubMed
-
- Andersen ND, Nielsen BE, Corradi J, Tolosa MF, Feuerbach D, Arias HR, Bouzat C, 2016. Exploring the positive allosteric modulation of human alpha7 nicotinic receptors from a single-channel perspective. Neuropharmacology 107, 189–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

