Time to Sustained Improvement in Bowel Movement Frequency with Telotristat Ethyl: Analyses of Phase III Studies in Carcinoid Syndrome
- PMID: 32146619
- PMCID: PMC7714089
- DOI: 10.1007/s12029-020-00375-2
Time to Sustained Improvement in Bowel Movement Frequency with Telotristat Ethyl: Analyses of Phase III Studies in Carcinoid Syndrome
Abstract
Background: Telotristat ethyl is approved to treat carcinoid syndrome diarrhea in combination with somatostatin analogs. In TELESTAR and TELECAST phase III studies, patients with carcinoid syndrome received telotristat ethyl 250 or 500 mg 3 times per day (tid) or placebo tid in addition to somatostatin analogs. The aim of this prespecified analysis was to examine the time to reductions in bowel movements (BMs) in the TELESTAR and TELECAST studies using survival analysis methods.
Methods: First occurrence of sustained response was defined as the time to the first day of 2 consecutive weeks with a mean BM frequency improvement of ≥ 30% from baseline during the 12-week double-blind treatment periods. Time to first ≥ 30% worsening in BM frequency was also measured. Treatments were compared with the log-rank test; Cox regression models provided point and confidence interval estimates of the hazard ratios for each trial.
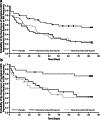
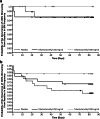
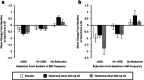
Results: In TELESTAR and TELECAST, majority of patients (69%) on telotristat ethyl experienced a sustained ≥ 30% improvement in BM frequency. The median time to sustained reduction of at least 30% in BM frequency was significantly faster (fewer days to onset) for telotristat ethyl compared with placebo in both TELESTAR (250 mg, HR = 2.3 [95% CI, 1.3-4.1, P = 0.004]; 500 mg, HR = 2.2 [95% CI, 1.2-3.9, P = 0.009]) and TELECAST (250 mg, HR = 3.9 [95% CI, 1.6-11.1, P = 0.003]; 500 mg, HR = 4.2 [95% CI, 1.7-11.7, P = 0.002]). In TELECAST, 42% of patients on placebo experienced sustained worsening in BM frequency compared with 20% on telotristat ethyl; no significant difference was observed in TELESTAR.
Conclusion: The time of onset of sustained BM frequency improvement mean and range are important when considering use of telotristat ethyl in patients with carcinoid syndrome diarrhea. Telotristat ethyl may also reduce sustained worsening in BM frequency.
Trial registration: ClinicalTrials.gov Identifiers: NCT01677910, NCT02063659.
Keywords: Diarrhea; Malignant carcinoid syndrome; Neuroendocrine tumors; Serotonin; Telotristat ethyl; Tryptophan hydroxylase.
Conflict of interest statement
Joseph S. Dillon: Lexicon Pharmaceuticals, Inc. (RF); Matthew H. Kulke: Lexicon Pharmaceuticals, Inc. (C/A), Ipsen Bioscience (C/A), Novartis Pharmaceuticals (C/A); Dieter Hörsch: Lexicon Pharmaceuticals, Inc. (H), Ipsen Pharmaceuticals, Inc. (RF, H); Lowell B. Anthony: Lexicon Pharmaceuticals, Inc. (RF, H); Richard R. P. Warner: Lexicon Pharmaceuticals, Inc. (RF); Emily Bergsland: UpToDate (IP), Novartis (RF); Staffan Welin: Ipsen (C/A), Novartis (C/A); Pamela L. Kunz: Lexicon Pharmaceuticals, Inc. (C/A), Advanced Accelerator Applications (RF), Genentech (RF), Ipsen (RF), Merck (RF), Incyte (RF); Chad McKee: Lexicon Pharmaceuticals, Inc. (E, OI); Pablo Lapuerta: Lexicon Pharmaceuticals, Inc. (E, OI); Phillip Banks: Lexicon Pharmaceuticals, Inc. (E, OI); Marianne Pavel: Novartis Pharmaceuticals (RF, H), Ipsen (RF, H), Lexicon Pharmaceuticals, Inc. (H), Pfizer, Inc. (H). Thomas M. O’Dorisio indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Figures

References
-
- Ruszniewski P, Ish-Shalom S, Wymenga M, O'Toole D, Arnold R, Tomassetti P, Bax N, Caplin M, Eriksson B, Glaser B, Ducreux M, Lombard-Bohas C, de Herder WW, Delle Fave G, Reed N, Seitz JF, van Cutsem E, Grossman A, Rougier P, Schmidt W, Wiedenmann B. Rapid and sustained relief from the symptoms of carcinoid syndrome: results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology. 2004;80:244–251. doi: 10.1159/000082875. - DOI - PubMed
-
- Lexicon Pharmaceuticals Inc. Xermelo® (telotristat ethyl) [prescribing information]. Lexicon Pharmaceuticals, Inc., The Woodlands, TX, 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208794s000lbl.pdf. Accessed February 20, 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
