Rates of Potentially Inappropriate Dosing of Direct-Acting Oral Anticoagulants and Associations With Geriatric Conditions Among Older Patients With Atrial Fibrillation: The SAGE-AF Study
- PMID: 32146898
- PMCID: PMC7335533
- DOI: 10.1161/JAHA.119.014108
Rates of Potentially Inappropriate Dosing of Direct-Acting Oral Anticoagulants and Associations With Geriatric Conditions Among Older Patients With Atrial Fibrillation: The SAGE-AF Study
Abstract
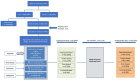
Background Direct-acting oral anticoagulant (DOAC) dosing guidelines for atrial fibrillation recommend dose alteration based on age, renal function, body weight, and drug-drug interactions. There is paucity of data describing the frequency and factors associated with prescription of potentially inappropriate doses. Methods and Results In the ongoing SAGE-AF (Systematic Assessment of Geriatric Elements in Atrial Fibrillation) study, we performed geriatric assessments (frailty, cognitive impairment, sensory impairments, social isolation, and depression) for participants with atrial fibrillation (age ≥65 years, CHA2DS2VASc ≥2, no anticoagulant contraindications). We developed an algorithm to analyze DOAC dose appropriateness accounting for drug-drug interactions, age, renal function, and body weight. We also examined whether geriatric impairments were related to inappropriate dosing. Of 1064 patients prescribed anticoagulants, 460 received a DOAC. Participants were aged 74±7 years, 49% were women, and 82% were white. A quarter (23%; n=105) of participants received inappropriate DOAC dose, of whom 82 (78%) were underdosed and 23 (22%) were overdosed. Among participants receiving an inappropriate dose, 12 (11%) were identified using the drug-drug interactions criteria and would have otherwise been misclassified. In multivariable regression analyses, older age, higher CHA2DS2VASc score, and history of renal failure were associated with inappropriate DOAC dosing (P<0.05). Geriatric conditions were not associated with inappropriate dosing. Conclusions In this cohort, over 20% of older patients with atrial fibrillation treated with DOACs were prescribed an inappropriate dose, with most being underdosed. Drug-drug interactions were common. Factors that influence prescription of guideline-nonadherent doses may be perception of higher bleeding risk or presence of renal failure in addition to lack of familiarity with dosing guidelines.
Keywords: anticoagulant; atrial fibrillation; geriatrics; off‐label dosing.
Figures

References
-
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. - PubMed
-
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. - PubMed
-
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. - PubMed
-
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; for the ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

