Phenotypic and Functional Changes in Peripheral Blood Natural Killer Cells in Crohn Disease Patients
- PMID: 32148442
- PMCID: PMC7049869
- DOI: 10.1155/2020/6401969
Phenotypic and Functional Changes in Peripheral Blood Natural Killer Cells in Crohn Disease Patients
Abstract
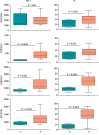
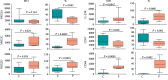
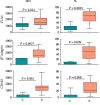
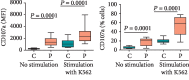
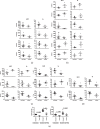
We investigated activation status, cytotoxic potential, and gut homing ability of the peripheral blood Natural Killer (NK) cells in Crohn disease (CD) patients. For this purpose, we compared the expression of different activating and inhibitory receptors (KIR and non-KIR) and integrins on NK cells as well as their recent degranulation history between the patients and age-matched healthy controls. The study was conducted using freshly obtained peripheral blood samples from the study participants. Multiple color flow cytometry was used for these determinations. Our results show that NK cells from treatment-naïve CD patients expressed higher levels of activating KIR as well as other non-KIR activating receptors vis-à-vis healthy controls. They also showed increased frequencies of the cells expressing these receptors. The expression of several KIR and non-KIR inhibitory receptors tended to decrease compared with the cells from healthy donors. NK cells from the patients also expressed increased levels of different gut-homing integrin molecules and showed a history of increased recent degranulation events both constitutively and in response to their in vitro stimulation. Furthermore, treatment of the patients tended to reverse these NK cell changes. Our results demonstrate unequivocally, for the first time, that peripheral blood NK cells in treatment-naïve CD patients are more activated and are more poised to migrate to the gut compared to their counterpart cells from healthy individuals. Moreover, they show that treatment of the patients tends to normalize their NK cells. The results suggest that NK cells are very likely to play a role in the immunopathogenesis of Crohn disease.
Copyright © 2020 Suzanne Samarani et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

