The resolution revolution in cryoEM requires high-quality sample preparation: a rapid pipeline to a high-resolution map of yeast fatty acid synthase
- PMID: 32148850
- PMCID: PMC7055384
- DOI: 10.1107/S2052252519017366
The resolution revolution in cryoEM requires high-quality sample preparation: a rapid pipeline to a high-resolution map of yeast fatty acid synthase
Abstract
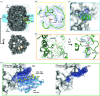
Single-particle electron cryo-microscopy (cryoEM) has undergone a 'resolution revolution' that makes it possible to characterize megadalton (MDa) complexes at atomic resolution without crystals. To fully exploit the new opportunities in molecular microscopy, new procedures for the cloning, expression and purification of macromolecular complexes need to be explored. Macromolecular assemblies are often unstable, and invasive construct design or inadequate purification conditions and sample-preparation methods can result in disassembly or denaturation. The structure of the 2.6 MDa yeast fatty acid synthase (FAS) has been studied by electron microscopy since the 1960s. Here, a new, streamlined protocol for the rapid production of purified yeast FAS for structure determination by high-resolution cryoEM is reported. Together with a companion protocol for preparing cryoEM specimens on a hydrophilized graphene layer, the new protocol yielded a 3.1 Å resolution map of yeast FAS from 15 000 automatically picked particles within a day. The high map quality enabled a complete atomic model of an intact fungal FAS to be built.
Keywords: 3D reconstruction and image processing; cryo-electron microscopy; macromolecular machines; protein structure; purification of protein complexes; single-particle cryoEM; yeast fatty acid synthase.
© Mirko Joppe et al. 2020.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

