Driving blind: instituting SEP-1 without high quality outcomes data
- PMID: 32148923
- PMCID: PMC7024755
- DOI: 10.21037/jtd.2019.12.100
Driving blind: instituting SEP-1 without high quality outcomes data
Erratum in
-
Erratum to driving blind: instituting SEP-1 without high quality outcomes data.J Thorac Dis. 2021 Jun;13(6):3932-3933. doi: 10.21037/jtd-2021-28. J Thorac Dis. 2021. PMID: 34277085 Free PMC article.
Abstract
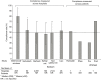
In 2015, the Centers for Medicare and Medicaid Services (CMS) instituted an all-or-none sepsis performance measure bundle (SEP-1) to promote high-quality, cost-effective care. Systematic reviews demonstrated only low-quality evidence supporting most of SEP-1's interventions. CMS has removed some but not all of these unproven components. The current SEP-1 version requires patients with suspected sepsis have a lactate level, blood cultures, broad-spectrum antibiotics and, if hypotensive, a fixed 30 mL/kg fluid infusion within 3 hours, and a repeat lactate if initially elevated within 6 hours. Experts have continued to raise concerns that SEP-1 remains overly prescriptive, lacks a sound scientific basis and presents risks (overuse of antibiotics and inappropriate fluids not titrated to need). To incentivize compliance with SEP-1, CMS now publicly publishes how often hospitals complete all interventions in individual patients. However, compliance measured across hospitals (5 studies, 48-2,851 hospitals) or patients (three studies, 110-851 patients) has been low (approximately 50%) which is not surprising given SEP-1's lack of scientific basis. The largest observational study (1,738 patients) reporting survival rates employing SEP-1 found they were not significantly improved with the measure (P=0.53) as did the next largest study (851 patients, adjusted survival odds ratio 1.36, 95% CI, 0.85 to 2.18). Two smaller observational studies (158 and 450 patients) reported SEP-1 improved unadjusted survival (P≤0.05) but were confounded either by baseline imbalances or by simultaneous introduction of a code sepsis protocol to improve compliance. Regardless, retrospective studies have well known biases related to non-randomized designs, uncontrolled data collection and failure to adjust for unrecognized influential variables. Such low-quality science should not be the basis for a national mandate compelling care for a rapidly lethal disease with a high mortality rate. Instead, SEP-1 should be based on high quality reproducible evidence from randomized controlled trials (RCT) demonstrating its benefit and thereby safety. Otherwise we risk not only doing harm but standardizing it.
Keywords: Sepsis; bundle; management; performance measure; septic shock; treatment.
2020 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
Comment in
-
Regarding Journal of Thoracic Disease Vol 12, Supplement 1 (February 2020) (Sepsis: Science and Fiction)-Driving blind: instituting SEP-1 without high quality outcomes data.J Thorac Dis. 2021 Jun;13(6):3930-3931. doi: 10.21037/jtd-2021-29. J Thorac Dis. 2021. PMID: 34277084 Free PMC article. No abstract available.
References
-
- Torio C, Andrews R. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. Agency for Healthcare Research and Quality 2013. - PubMed
-
- CMS to Improve Quality of Care during Hospital Inpatient Stays. CMS.gov Fact Sheet. 2014. Available online: https://www.cms.gov/newsroom/fact-sheets/cms-improve-quality-care-during.... 26 November 2019.
-
- Hospital Inpatient Quality Reporting (IQR) Program. CMS.gov. Available online: https://www.qualitynet.org/inpatient/iqr
-
- Hospital Inpatient Quality Reporting Program. CMS.gov. 2017. Available online: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Inst...
Publication types
LinkOut - more resources
Full Text Sources
