Sulfonamide-Based Azaheterocyclic Schiff Base Derivatives as Potential Carbonic Anhydrase Inhibitors: Synthesis, Cytotoxicity, and Enzyme Inhibitory Kinetics
- PMID: 32149140
- PMCID: PMC7054763
- DOI: 10.1155/2020/8104107
Sulfonamide-Based Azaheterocyclic Schiff Base Derivatives as Potential Carbonic Anhydrase Inhibitors: Synthesis, Cytotoxicity, and Enzyme Inhibitory Kinetics
Abstract
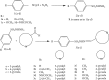
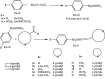
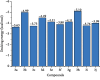
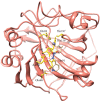
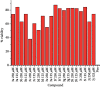
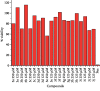
A series of sulfonamide-bearing azaheterocyclic Schiff base derivatives 3(a-j) were synthesized as carbonic anhydrase inhibitors. The substituted benzene sulfonyl chlorides 1(a-d) were reacted with N2H4 to get aromatic sulfonyl hydrazides 2(a-d). The intermediate hydrazides 2(a-d) were treated with substituted aldehydes to afford azaheterocyclic sulfonamide Schiff bases 3(a-j). The spectral data of synthesized compounds confirmed the formation of the final products. The inhibitory effects of 3(a-j) on carbonic anhydrase activity were determined, and it was found that derivative 3c exhibited the most potent activity with IC500.84 ± 0.12 μM among all other derivatives and is also more active than standard acetazolamide (IC500.91 ± 0.12). The enzyme inhibitory kinetics results determined by Lineweaver-Burk plots revealed that compound 3c inhibits the enzyme by noncompetitive mode of inhibition with K i value 8.6 μM. The molecular docking investigations of the synthesized analogues 3(a-j) were evaluated which assured that synthesized compounds bind well inside the active binding site of the target enzyme. Cytotoxicity on human keratinocyte (HaCaT) and MCF-7 cell lines was performed, and it was found that most of the synthesized analogues were nontoxic on these cell lines and the toxic effects follow the dose-dependent manner. Based on our investigations, it was suggested that analogue 3c may serve as core structure to project carbonic anhydrase inhibitors with greater potency.
Copyright © 2020 Mujahid Abas et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Kannan K. K., Notstrand B., Fridborg K., Lovgren S., Ohlsson A., Petef M. Crystal structure of human erythrocyte carbonic anhydrase B. Three-dimensional structure at a nominal 2.2-A resolution. Proceedings of the National Academy of Sciences. 1975;72(1):51–55. doi: 10.1073/pnas.72.1.51. - DOI - PMC - PubMed
-
- Maresca A., Scozzafava A., Vullo D., Supuran C. T. Dihalogenated sulfanilamides and benzolamides are effective inhibitors of the three β-class carbonic anhydrases from Mycobacterium tuberculosis. Journal of Enzyme Inhibition and Medicinal Chemistry. 2013;28(2):384–387. doi: 10.3109/14756366.2011.645539. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

