A Solution with Ginseng Saponins and Selenium as Vaccine Diluent to Increase Th1/Th2 Immune Responses in Mice
- PMID: 32149156
- PMCID: PMC7054799
- DOI: 10.1155/2020/2714257
A Solution with Ginseng Saponins and Selenium as Vaccine Diluent to Increase Th1/Th2 Immune Responses in Mice
Abstract
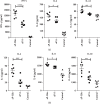
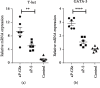
Pseudorabies is an important infectious disease of swine, and immunization using attenuated pseudorabies virus (aPrV) vaccine is a routine practice to control this disease in swine herds. This study was to evaluate a saline solution containing ginseng stem-leaf saponins (GSLS) and sodium selenite (Se) as a vaccine adjuvant for its enhancement of immune response to aPrV vaccine. The results showed that aPrV vaccine diluted with saline containing GSLS-Se (aP-GSe) induced significantly higher immune responses than that of the vaccine diluted with saline alone (aP-S). The aP-GSe promoted higher production of gB-specific IgG, IgG1, and IgG2a, neutralizing antibody titers, secretion of Th1-type (IFN-γ, IL-2, IL-12), and Th2-type (IL-4, IL-6, IL-10) cytokines, and upregulated the T-bet/GATA-3 mRNA expression when compared to aP-S. In addition, cytolytic activity of NK cells, lymphocyte proliferation, and CD4+/CD8+ ratio was also significantly increased by aP-GSe. More importantly, aP-GSe conferred a much higher resistance of mice to a field virulent pseudorabies virus (fPrV) challenge. As the present study was conducted in mice, further study is required to evaluate the aP-GSe to improve the vaccination against PrV in swine.
Copyright © 2020 Yong Wang et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ginseng stem-leaf saponins in combination with selenium enhance immune responses to an attenuated pseudorabies virus vaccine.Microbiol Immunol. 2019 Jul;63(7):269-279. doi: 10.1111/1348-0421.12715. Epub 2019 Jun 27. Microbiol Immunol. 2019. PMID: 31141221
-
Improved immune response to an attenuated pseudorabies virus vaccine by ginseng stem-leaf saponins (GSLS) in combination with thimerosal (TS).Antiviral Res. 2016 Aug;132:92-8. doi: 10.1016/j.antiviral.2016.05.018. Epub 2016 May 27. Antiviral Res. 2016. PMID: 27241688
-
Ginseng Stem-Leaf Saponins in Combination with Selenium Promote the Immune Response in Neonatal Mice with Maternal Antibody.Vaccines (Basel). 2020 Dec 11;8(4):755. doi: 10.3390/vaccines8040755. Vaccines (Basel). 2020. PMID: 33322647 Free PMC article.
-
Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine.Adv Vet Med. 1999;41:447-61. doi: 10.1016/s0065-3519(99)80034-2. Adv Vet Med. 1999. PMID: 9890035 Review.
-
Design and application of nanoparticles as vaccine adjuvants against human corona virus infection.J Inorg Biochem. 2021 Jun;219:111454. doi: 10.1016/j.jinorgbio.2021.111454. Epub 2021 Mar 29. J Inorg Biochem. 2021. PMID: 33878530 Free PMC article. Review.
Cited by
-
A single dose of Astragalus saponins adjuvanted inactivated vaccine for pseudorabies virus protected mice against lethal challenge.Front Vet Sci. 2022 Nov 21;9:1036161. doi: 10.3389/fvets.2022.1036161. eCollection 2022. Front Vet Sci. 2022. PMID: 36478947 Free PMC article.
-
Immunomodulatory and Anticancer Activities of Barley Bran Grown in Jordan: An in vitro and in vivo Study.Front Nutr. 2022 May 18;9:838373. doi: 10.3389/fnut.2022.838373. eCollection 2022. Front Nutr. 2022. PMID: 35662936 Free PMC article.
-
Immunomodulatory functional foods and their molecular mechanisms.Exp Mol Med. 2022 Jan;54(1):1-11. doi: 10.1038/s12276-022-00724-0. Epub 2022 Jan 25. Exp Mol Med. 2022. PMID: 35079119 Free PMC article. Review.
-
Membrane fusion, potential threats, and natural antiviral drugs of pseudorabies virus.Vet Res. 2023 May 2;54(1):39. doi: 10.1186/s13567-023-01171-z. Vet Res. 2023. PMID: 37131259 Free PMC article. Review.
-
Effects of Casein Phosphopeptide-Selenium Complex on the Immune Functions in Beagle Dogs.Animals (Basel). 2022 Aug 10;12(16):2037. doi: 10.3390/ani12162037. Animals (Basel). 2022. PMID: 36009627 Free PMC article.
References
-
- Constable P. D., Hinchcliff K. W., Done S. H., Grünberg W. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. In: Constable P. D., editor. Diseases of the Nervous System. Elsevier; 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

