ZnO-Modified g-C3N4: A Potential Photocatalyst for Environmental Application
- PMID: 32149209
- PMCID: PMC7057336
- DOI: 10.1021/acsomega.9b02688
ZnO-Modified g-C3N4: A Potential Photocatalyst for Environmental Application
Abstract
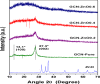
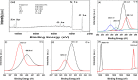
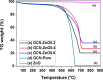
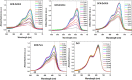
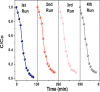
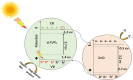
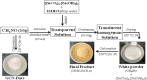
Solar energy-driven practices using semiconducting materials is an ideal approach toward wastewater remediation. In order to attain a superior photocatalyst, a composite of g-C3N4 and ZnO (GCN-ZnO) has been prepared by one-step thermal polymerization of urea and zinc carbonate basic dihydrate [ZnNO3]2·[Zn(OH)2]3. The GCN-ZnO0.4 sample showed an evolved morphology, increased surface area (116 m2 g-1), better visible light absorption ability, and reduced band gap in comparison to GCN-pure. The GCN-ZnO0.4 sample also showed enhanced adsorption and photocatalytic activity performance, resulting in an increased reaction rate value up to 3 times that of GCN-pure, which was attributed to the phenomenon of better separation of photogenerated charge carriers resulting because of heterojunction development among interfaces of GCN-pure and ZnO. In addition, the GCN-ZnO0.4 sample showed a decent stability for four cyclic runs and established its potential use for abatement of organic wastewater pollutants in comparison to GCN-pure.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Patsoura A.; Kondarides D. I.; Verykios X. E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102. 10.1016/j.cattod.2007.03.028. - DOI
-
- Fageria P.; Gangopadhyay S.; Pande S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. 10.1039/c4ra03158j. - DOI
-
- Panchal P.; Paul D. R.; Sharma A.; Hooda D.; Yadav R.; Meena P.; Nehra S. P. Phytoextract mediated ZnO/MgO nanocomposites for photocatalytic and antibacterial activities. J. Photochem. Photobiol., A 2019, 385, 112049.10.1016/j.jphotochem.2019.112049. - DOI
-
- Safaei J.; Mohamed N. A.; Mohamad Noh M. F.; Soh M. F.; Ludin N. A.; Ibrahim M. A.; Roslam Wan Isahak W. N.; Mat Teridi M. A. Graphitic carbon nitride (g-C3N4) electrodes for energy conversion and storage: a review on photoelectrochemical water splitting, solar cells and supercapacitors. J. Mater. Chem. A 2018, 6, 22346.10.1039/c8ta08001a. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
