Measuring Cellular Ion Transport by Magnetoencephalography
- PMID: 32149229
- PMCID: PMC7057328
- DOI: 10.1021/acsomega.9b03589
Measuring Cellular Ion Transport by Magnetoencephalography
Abstract
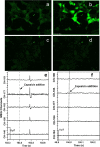
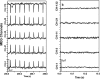
The cellular-level process of ion transport is known to generate a magnetic field. A noninvasive magnetoencephalography (MEG) technique was used to measure the magnetic field emanating from HeLa, HEK293, and H9c2(2-1) rat cardiac cells. The addition of a nonlethal dose of ionomycin to HeLa and capsaicin to TRPV1-expressing HEK293 cells resulted in a sudden change in the magnetic field signal consistent with Ca2+ influx, which was also observed by confocal fluorescence microscopy under the same conditions. In contrast, addition of capsaicin to TRPV1-expressing HEK293 cells containing an optimum amount of a TRPV1 antagonist (ruthenium red), resulted in no detectable magnetic or fluorescent signals. These signals confirmed that the measured MEG signals are due to cellular ion transport through the cell membrane. In general, there is evidence that ion channel/transporter activation and ionic flux are linked to cancer. Therefore, our work suggests that MEG could represent a noninvasive method for detecting cancer.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous
