Click Chemistry-Based Two-Component System for Efficient Inhibition of Human Immunodeficiency Virus (HIV) Reverse Transcriptase (RT)
- PMID: 32149246
- PMCID: PMC7057708
- DOI: 10.1021/acsomega.9b03942
Click Chemistry-Based Two-Component System for Efficient Inhibition of Human Immunodeficiency Virus (HIV) Reverse Transcriptase (RT)
Abstract
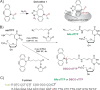
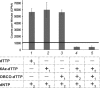
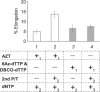
We synthesized two dTTP analogues for copper-free "click" chemistry-coupling in the active sites of DNA polymerases. We found that in the presence of both analogues, human immunodeficiency virus (HIV) reverse transcriptase (RT) activity was suppressed by up to 93%. This inhibitory effect was not recovered by an excess amount of primer-template unlike that for a conventional HIV RT inhibitor, azidothymidine. This finding may become the basis for the development of efficient in vivo inhibitors of HIV RT and other DNA polymerases.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Wainberg M. A.; Sawyer J. P.; Montaner J. S.; Murphy R. L.; Kuritzkes D. R.; Raffi F. Challenges for the clinical development of new nucleoside reverse transcriptase inhibitors for HIV infection. Antiviral Ther. 2005, 10, 13–28. - PubMed
-
- Meyer P. R.; Matsuura S. E.; Schinazi R. F.; So A. G.; Scott W. A. Differential removal of thymidine nucleotide analogues from blocked DNA chains by human immunodeficiency virus reverse transcriptase in the presence of physiological concentrations of 2′-deoxynucleoside triphosphates. Antimicrob. Agents Chemother. 2000, 44, 3465–3472. 10.1128/AAC.44.12.3465-3472.2000. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

