Rapid regulation of photosynthetic light harvesting in the absence of minor antenna and reaction centre complexes
- PMID: 32149343
- PMCID: PMC7307847
- DOI: 10.1093/jxb/eraa126
Rapid regulation of photosynthetic light harvesting in the absence of minor antenna and reaction centre complexes
Abstract
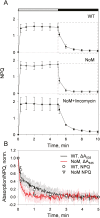
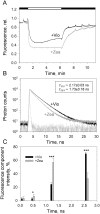
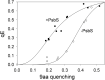
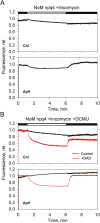
Plants are subject to dramatic fluctuations in the intensity of sunlight throughout the day. When the photosynthetic machinery is exposed to high light, photons are absorbed in excess, potentially leading to oxidative damage of its delicate membrane components. A photoprotective molecular process called non-photochemical quenching (NPQ) is the fastest response carried out in the thylakoid membranes to harmlessly dissipate excess light energy. Despite having been intensely studied, the site and mechanism of this essential regulatory process are still debated. Here, we show that the main NPQ component called energy-dependent quenching (qE) is present in plants with photosynthetic membranes largely enriched in the major trimeric light-harvesting complex (LHC) II, while being deprived of all minor LHCs and most photosystem core proteins. This fast and reversible quenching depends upon thylakoid lumen acidification (ΔpH). Enhancing ΔpH amplifies the extent of the quenching and restores qE in the membranes lacking PSII subunit S protein (PsbS), whereas the carotenoid zeaxanthin modulates the kinetics and amplitude of the quenching. These findings highlight the self-regulatory properties of the photosynthetic light-harvesting membranes in vivo, where the ability to switch reversibly between the harvesting and dissipative states is an intrinsic property of the major LHCII.
Keywords: Chlorophyll fluorescence; LHCII; NPQ; proton gradient; qE; zeaxanthin.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



Comment in
-
Just the essentials: photoprotective energy dissipation pared-down.J Exp Bot. 2020 Jun 22;71(12):3380-3382. doi: 10.1093/jxb/eraa164. J Exp Bot. 2020. PMID: 32569382 No abstract available.
Similar articles
-
The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching.Nat Plants. 2017 Jan 30;3:16225. doi: 10.1038/nplants.2016.225. Nat Plants. 2017. PMID: 28134919
-
The causes of altered chlorophyll fluorescence quenching induction in the Arabidopsis mutant lacking all minor antenna complexes.Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):666-675. doi: 10.1016/j.bbabio.2018.03.005. Epub 2018 Mar 13. Biochim Biophys Acta Bioenerg. 2018. PMID: 29548769
-
Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced DeltapH.J Biol Chem. 2011 Jun 3;286(22):19973-81. doi: 10.1074/jbc.M111.237255. Epub 2011 Apr 7. J Biol Chem. 2011. PMID: 21474447 Free PMC article.
-
Energy-Dependent Non-Photochemical Quenching: PsbS, LhcSR, and Other Players.Biochemistry (Mosc). 2025 Jan;90(1):44-60. doi: 10.1134/S000629792460371X. Biochemistry (Mosc). 2025. PMID: 40058973 Review.
-
The Mechanism of Non-Photochemical Quenching in Plants: Localization and Driving Forces.Plant Cell Physiol. 2021 Oct 29;62(7):1063-1072. doi: 10.1093/pcp/pcaa155. Plant Cell Physiol. 2021. PMID: 33351147 Review.
Cited by
-
Energetic driving force for LHCII clustering in plant membranes.Sci Adv. 2023 Dec 22;9(51):eadj0807. doi: 10.1126/sciadv.adj0807. Epub 2023 Dec 22. Sci Adv. 2023. PMID: 38134273 Free PMC article.
-
Towards spruce-type photosystem II: consequences of the loss of light-harvesting proteins LHCB3 and LHCB6 in Arabidopsis.Plant Physiol. 2021 Dec 4;187(4):2691-2715. doi: 10.1093/plphys/kiab396. Plant Physiol. 2021. PMID: 34618099 Free PMC article.
-
Ferredoxin C2 is required for chlorophyll biosynthesis and accumulation of photosynthetic antennae in Arabidopsis.Plant Cell Environ. 2023 Nov;46(11):3287-3304. doi: 10.1111/pce.14667. Epub 2023 Jul 10. Plant Cell Environ. 2023. PMID: 37427830 Free PMC article.
-
How the pH Controls Photoprotection in the Light-Harvesting Complex of Mosses.J Am Chem Soc. 2023 Apr 5;145(13):7482-7494. doi: 10.1021/jacs.3c00377. Epub 2023 Mar 24. J Am Chem Soc. 2023. PMID: 36961522 Free PMC article.
-
A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein.Photosynth Res. 2023 Apr;156(1):163-177. doi: 10.1007/s11120-022-00935-6. Epub 2022 Jul 10. Photosynth Res. 2023. PMID: 35816266 Free PMC article. Review.
References
-
- Akhtar P, Görföl F, Garab G, Lambrev PH. 2019. Dependence of chlorophyll fluorescence quenching on the lipid-to-protein ratio in reconstituted light-harvesting complex II membranes containing lipid labels. Chemical Physics 522, 242–248.
-
- Anderson J, Chow W, Goodchild D. 1988. Thylakoid membrane organisation in sun/shade acclimation. Functional Plant Biology 15, 11–26.
-
- Aro EM, Virgin I, Andersson B. 1993. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta 1143, 113–134. - PubMed
-
- Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR. 2008. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. Journal of Biological Chemistry 283, 3550–3558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

