Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model
- PMID: 32149769
- PMCID: PMC7147283
- DOI: 10.1097/CM9.0000000000000797
Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model
Abstract
Background: Medicines for the treatment of 2019-novel coronavirus (2019-nCoV) infections are urgently needed. However, drug screening using live 2019-nCoV requires high-level biosafety facilities, which imposes an obstacle for those institutions without such facilities or 2019-nCoV. This study aims to repurpose the clinically approved drugs for the treatment of coronavirus disease 2019 (COVID-19) in a 2019-nCoV-related coronavirus model.
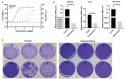
Methods: A 2019-nCoV-related pangolin coronavirus GX_P2V/pangolin/2017/Guangxi was described. Whether GX_P2V uses angiotensin-converting enzyme 2 (ACE2) as the cell receptor was investigated by using small interfering RNA (siRNA)-mediated silencing of ACE2. The pangolin coronavirus model was used to identify drug candidates for treating 2019-nCoV infection. Two libraries of 2406 clinically approved drugs were screened for their ability to inhibit cytopathic effects on Vero E6 cells by GX_P2V infection. The anti-viral activities and anti-viral mechanisms of potential drugs were further investigated. Viral yields of RNAs and infectious particles were quantified by quantitative real-time polymerase chain reaction (qRT-PCR) and plaque assay, respectively.
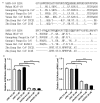
Results: The spike protein of coronavirus GX_P2V shares 92.2% amino acid identity with that of 2019-nCoV isolate Wuhan-hu-1, and uses ACE2 as the receptor for infection just like 2019-nCoV. Three drugs, including cepharanthine (CEP), selamectin, and mefloquine hydrochloride, exhibited complete inhibition of cytopathic effects in cell culture at 10 μmol/L. CEP demonstrated the most potent inhibition of GX_P2V infection, with a concentration for 50% of maximal effect [EC50] of 0.98 μmol/L. The viral RNA yield in cells treated with 10 μmol/L CEP was 15,393-fold lower than in cells without CEP treatment ([6.48 ± 0.02] × 10vs. 1.00 ± 0.12, t = 150.38, P < 0.001) at 72 h post-infection (p.i.). Plaque assays found no production of live viruses in media containing 10 μmol/L CEP at 48 h p.i. Furthermore, we found CEP had potent anti-viral activities against both viral entry (0.46 ± 0.12, vs.1.00 ± 0.37, t = 2.42, P < 0.05) and viral replication ([6.18 ± 0.95] × 10vs. 1.00 ± 0.43, t = 3.98, P < 0.05).
Conclusions: Our pangolin coronavirus GX_P2V is a workable model for 2019-nCoV research. CEP, selamectin, and mefloquine hydrochloride are potential drugs for treating 2019-nCoV infection. Our results strongly suggest that CEP is a wide-spectrum inhibitor of pan-betacoronavirus, and further study of CEP for treatment of 2019-nCoV infection is warranted.
Conflict of interest statement
None.
Figures

References
-
- Gorbalenya AE. Severe acute respiratory syndrome-related coronavirus: the species and its viruses, a statement of the Coronavirus Study Group. bioRxiv 2020; doi: 10.1101/2020.02.07.937862.
-
- Xiao K, Zhai J, Feng Y, Zhou N, Zhang X, Zou JJ, et al. Isolation and characterization of 2019-nCoV-like coronavirus from Malayan Pangolins. bioRxiv 2020; doi: 10.1101/2020.02.17.951335.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

