Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial
- PMID: 32149773
- PMCID: PMC7147272
- DOI: 10.1097/CM9.0000000000000791
Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial
Abstract
Background: At the end of 2019, a novel coronavirus outbreak causative organism has been subsequently designated the 2019 novel coronavirus (2019-nCoV). The effectiveness of adjunctive glucocorticoid therapy in the management of 2019-nCoV-infected patients with severe lower respiratory tract infections is not clear, and warrants further investigation.
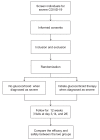
Methods: The present study will be conducted as an open-labeled, randomized, controlled trial. We will enrol 48 subjects from Chongqing Public Health Medical Center. Each eligible subject will be assigned to an intervention group (methylprednisolone via intravenous injection at a dose of 1-2 mg/kg/day for 3 days) or a control group (no glucocorticoid use) randomly, at a 1:1 ratio. Subjects in both groups will be invited for 28 days of follow-up which will be scheduled at four consecutive visit points. We will use the clinical improvement rate as our primary endpoint. Secondary endpoints include the timing of clinical improvement after intervention, duration of mechanical ventilation, duration of hospitalization, overall incidence of adverse events, as well as rate of adverse events at each visit, and mortality at 2 and 4 weeks.
Discussion: The present coronavirus outbreak is the third serious global coronavirus outbreak in the past two decades. Oral and parenteral glucocorticoids have been used in the management of severe respiratory symptoms in coronavirus-infected patients in the past. However, there remains no definitive evidence in the literature for or against the utilization of systemic glucocorticoids in seriously ill patients with coronavirus-related severe respiratory disease, or indeed in other types of severe respiratory disease. In this study, we hope to discover evidence either supporting or opposing the systemic therapeutic administration of glucocorticoids in patients with severe coronavirus disease 2019.
Trial registration: ClinicalTrials.gov, ChiCTR2000029386, http://www.chictr.org.cn/showproj.aspx?proj=48777.
Conflict of interest statement
None.
Figures
Comment in
-
The quality of the reported sample size calculation in clinical trials on COVID-19 patients indexed in PubMed.Eur J Intern Med. 2020 Jul;77:139-140. doi: 10.1016/j.ejim.2020.04.057. Epub 2020 Apr 30. Eur J Intern Med. 2020. PMID: 32414641 Free PMC article. No abstract available.
References
-
- WHO. Summary of probable SARS cases with onset of illness from November 1, 2002 to July 31, 2003. WHO, 2003. Available from: https://www.who.int/csr/sars/country/table2004_04_21/en/. [Accessed on February 2, 2020]
-
- WHO. Middle East respiratory syndrome coronavirus (MERS-CoV). 2019. Available from: http://www.who.int/emergencies/mers-cov/en/. [Accessed on February 2, 2020]
-
- WHO. Novel Coronavirus(2019-nCoV)SITUATION REPORT-121 JANUARY 2020. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... [Accessed January 20, 2020]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous