Association Between Renin-Angiotensin System Blockade Discontinuation and All-Cause Mortality Among Persons With Low Estimated Glomerular Filtration Rate
- PMID: 32150237
- PMCID: PMC7063544
- DOI: 10.1001/jamainternmed.2020.0193
Association Between Renin-Angiotensin System Blockade Discontinuation and All-Cause Mortality Among Persons With Low Estimated Glomerular Filtration Rate
Abstract
Importance: It is uncertain whether and when angiotensin-converting enzyme inhibitor (ACE-I) and angiotensin II receptor blocker (ARB) treatment should be discontinued in individuals with low estimated glomerular filtration rate (eGFR).
Objective: To investigate the association of ACE-I or ARB therapy discontinuation after eGFR decreases to below 30 mL/min/1.73 m2 with the risk of mortality, major adverse cardiovascular events (MACE), and end-stage kidney disease (ESKD).
Design, setting, and participants: This retrospective, propensity score-matched cohort study included 3909 patients from an integrated health care system that served rural areas of central and northeastern Pennsylvania. Patients who initiated ACE-I or ARB therapy from January 1, 2004, to December 31, 2018, and had an eGFR decrease to below 30 mL/min/1.73 m2 during therapy were enrolled, with follow-up until January 25, 2019.
Exposures: Individuals were classified based on whether they discontinued ACE-I or ARB therapy within 6 months after an eGFR decrease to below 30 mL/min/1.73 m2.
Main outcomes and measures: The association between ACE-I or ARB therapy discontinuation and mortality during the subsequent 5 years was assessed using multivariable Cox proportional hazards regression models, adjusting for patient characteristics at the time of the eGFR decrease in a propensity score-matched sample. Secondary outcomes included MACE and ESKD.
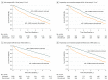
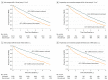
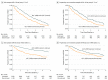
Results: Of the 3909 individuals receiving ACE-I or ARB treatment who experienced an eGFR decrease to below 30 mL/min/1.73 m2 (2406 [61.6%] female; mean [SD] age, 73.7 [12.6] years), 1235 discontinued ACE-I or ARB therapy within 6 months after the eGFR decrease and 2674 did not discontinue therapy. A total of 434 patients (35.1%) who discontinued ACE-I or ARB therapy and 786 (29.4%) who did not discontinue therapy died during a median follow-up of 2.9 years (interquartile range, 1.3-5.0 years). In the propensity score-matched sample of 2410 individuals, ACE-I or ARB therapy discontinuation was associated with a higher risk of mortality (hazard ratio [HR], 1.39; 95% CI, 1.20-1.60]) and MACE (HR, 1.37; 95% CI, 1.20-1.56), but no statistically significant difference in the risk of ESKD was found (HR, 1.19; 95% CI, 0.86-1.65).
Conclusions and relevance: The findings suggest that continuing ACE-I or ARB therapy in patients with declining kidney function may be associated with cardiovascular benefit without excessive harm of ESKD.
Conflict of interest statement
Figures
Comment in
-
Continuation of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in the Face of Kidney Disease Progression-Safe and Possibly Life Saving.JAMA Intern Med. 2020 May 1;180(5):727. doi: 10.1001/jamainternmed.2020.0300. JAMA Intern Med. 2020. PMID: 32150238 No abstract available.
-
Should Renin-Angiotensin System Blockade Be Avoided in Patients With Declining Kidney Function?Am J Kidney Dis. 2020 Nov;76(5):739-741. doi: 10.1053/j.ajkd.2020.04.003. Epub 2020 May 12. Am J Kidney Dis. 2020. PMID: 32407751 No abstract available.
References
-
- Agodoa LY, Appel L, Bakris GL, et al. ; African American Study of Kidney Disease and Hypertension (AASK) Study Group . Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719-2728. doi: 10.1001/jama.285.21.2719 - DOI - PubMed
-
- Marin R, Ruilope LM, Aljama P, Aranda P, Segura J, Diez J; Investigators of the ESPIRAL Study; Efecto del tratamiento antihipertensivo Sobre la Progresión de la Insuficiencia RenAL en pacientes no diabéticos . A random comparison of fosinopril and nifedipine GITS in patients with primary renal disease. J Hypertens. 2001;19(10):1871-1876. doi: 10.1097/00004872-200110000-00023 - DOI - PubMed
-
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi: 10.1016/j.jacc.2017.11.006 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

