Comparison of Neuroprotective Effects of Monomethylfumarate to the Sigma 1 Receptor Ligand (+)-Pentazocine in a Murine Model of Retinitis Pigmentosa
- PMID: 32150247
- PMCID: PMC7401726
- DOI: 10.1167/iovs.61.3.5
Comparison of Neuroprotective Effects of Monomethylfumarate to the Sigma 1 Receptor Ligand (+)-Pentazocine in a Murine Model of Retinitis Pigmentosa
Abstract
Purpose: Activating the cell survival modulator sigma 1 receptor (Sig1R) delays cone photoreceptor cell loss in Pde6βrd10/J (rd10) mice, a model of retinitis pigmentosa. Beneficial effects are abrogated in rd10 mice lacking NRF2, implicating NRF2 as essential to Sig1R-mediated cone neuroprotection. Here we asked whether activation of NRF2 alone is sufficient to rescue cones in rd10 mice.
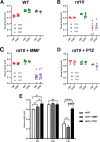
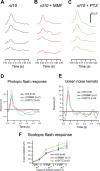
Methods: Expression of antioxidant genes was evaluated in 661W cells and in mouse retinas after treatment with monomethylfumarate (MMF), a potent NRF2 activator. Rd10 mice were administered MMF (50 mg/kg) or the Sig1R ligand (+)-pentazocine (PTZ; 0.5 mg/kg) intraperitoneally (every other day, P14-42). Mice were evaluated for visual acuity (optokinetic tracking response), retinal function (electroretinography) and architecture (SD-OCT); histologic retinal sections were evaluated morphometrically.
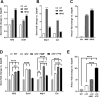
Results: MMF treatment increased Nrf2, Nqo1, Cat, Sod1, and Hmox1 expression in vitro and in vivo. Visual acuity of (+)-PTZ-treated rd10 mice was similar to wild-type mice; however, MMF treatment did not alter acuity compared with nontreated rd10 mice. Cone electroretinography b-wave amplitudes were greater in PTZ-treated than nontreated or MMF-treated rd10 mice. SD-OCT assessment of retinal thickness was greater in (+)-PTZ-treated mice versus nontreated or MMF-treated rd10 mice. Morphometric assessment of the outer nuclear layer revealed approximately 18 cells/100 µm retinal length in (+)-PTZ-treated rd10 mice, but only approximately 10 to 12 cells/100 µm in MMF-treated and nontreated rd10 retinas.
Conclusions: Activation of NRF2 using MMF, at least at our dosing regimen, is insufficient to attenuate catastrophic photoreceptor damage characteristic of rd10 mice. The data prompt investigation of additional mechanisms involved in Sig1R-mediated retinal neuroprotection.
Conflict of interest statement
Disclosure:
Figures


References
-
- Campochiaro PA, Mir TA. The mechanism of cone cell death in retinitis pigmentosa. Prog Retin Eye Res. 2018; 62: 24–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

