High Incidence of Herpes Zoster After Cord Blood Hematopoietic Cell Transplant Despite Longer Duration of Antiviral Prophylaxis
- PMID: 32150265
- PMCID: PMC8075034
- DOI: 10.1093/cid/ciaa222
High Incidence of Herpes Zoster After Cord Blood Hematopoietic Cell Transplant Despite Longer Duration of Antiviral Prophylaxis
Abstract
Background: Cord blood transplant (CBT) recipients have a high incidence of herpes zoster (HZ) in the context of short-term peritransplant antiviral prophylaxis. In 2009, international guidelines recommended HZ prophylaxis for at least 1 year after hematopoietic cell transplant. The impact of longer-term antiviral prophylaxis on HZ incidence after CBT is unknown.
Methods: We retrospectively analyzed varicella zoster virus (VZV)-seropositive CBT recipients who were transplanted between 2006 and 2016. We abstracted HZ events and other variables for up to 5 years post-CBT. We calculated the cumulative incidence of HZ and used Cox proportional hazards regression to identify variables associated with HZ.
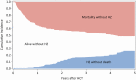
Results: The study cohort consisted of 227 patients. Among 1-year survivors, 91% were still receiving prophylaxis, for a median duration of 20.6 months. HZ occurred in 44 patients (19%) at a median of 23.6 months. The cumulative incidence of HZ by 1 year after CBT was 1.8% (95% confidence interval [CI], .1%-4%), but increased to 26% (95% CI, 19%-33%) by 5 years. In a multivariable analysis, acute graft-vs-host disease was associated with increased risk, whereas antiviral prophylaxis was associated with reduced risk for HZ (adjusted hazard ratio, 0.19 [95% CI, .09-.4]). There was no association between CD4+ T-cell counts at 1 year post-CBT and subsequent risk for HZ.
Conclusions: We found a high incidence of HZ after CBT despite antiviral prophylaxis for > 1 year. Based on these findings, we suggest longer duration of prophylaxis for HZ after CBT. Compliance with antiviral prophylaxis, VZV-specific immune monitoring, and vaccination to mitigate HZ after CBT also require further study.
Keywords: antiviral; cord blood; hematopoietic cell transplant; prophylaxis; varicella zoster virus.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Koc Y, Miller KB, Schenkein DP, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant 2000; 6:44–9. - PubMed
-
- Yagi T, Karasuno T, Hasegawa T, et al. Acute abdomen without cutaneous signs of varicella zoster virus infection as a late complication of allogeneic bone marrow transplantation: importance of empiric therapy with acyclovir. Bone Marrow Transplant 2000; 25:1003–5. - PubMed
-
- Grant RM, Weitzman SS, Sherman CG, Sirkin WL, Petric M, Tellier R. Fulminant disseminated varicella zoster virus infection without skin involvement. J Clin Virol 2002; 24:7–12. - PubMed
-
- Onozawa M, Hashino S, Haseyama Y, et al. Incidence and risk of postherpetic neuralgia after varicella zoster virus infection in hematopoietic cell transplantation recipients: Hokkaido Hematology Study Group. Biol Blood Marrow Transplant 2009; 15:724–9. - PubMed
-
- Kanbayashi Y, Matsumoto Y, Kuroda J, et al. Predicting risk factors for varicella zoster virus infection and postherpetic neuralgia after hematopoietic cell transplantation using ordered logistic regression analysis. Ann Hematol 2017; 96:311–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

