High Incidence of Herpes Zoster After Cord Blood Hematopoietic Cell Transplant Despite Longer Duration of Antiviral Prophylaxis
- PMID: 32150265
- PMCID: PMC8075034
- DOI: 10.1093/cid/ciaa222
High Incidence of Herpes Zoster After Cord Blood Hematopoietic Cell Transplant Despite Longer Duration of Antiviral Prophylaxis
Abstract
Background: Cord blood transplant (CBT) recipients have a high incidence of herpes zoster (HZ) in the context of short-term peritransplant antiviral prophylaxis. In 2009, international guidelines recommended HZ prophylaxis for at least 1 year after hematopoietic cell transplant. The impact of longer-term antiviral prophylaxis on HZ incidence after CBT is unknown.
Methods: We retrospectively analyzed varicella zoster virus (VZV)-seropositive CBT recipients who were transplanted between 2006 and 2016. We abstracted HZ events and other variables for up to 5 years post-CBT. We calculated the cumulative incidence of HZ and used Cox proportional hazards regression to identify variables associated with HZ.
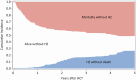
Results: The study cohort consisted of 227 patients. Among 1-year survivors, 91% were still receiving prophylaxis, for a median duration of 20.6 months. HZ occurred in 44 patients (19%) at a median of 23.6 months. The cumulative incidence of HZ by 1 year after CBT was 1.8% (95% confidence interval [CI], .1%-4%), but increased to 26% (95% CI, 19%-33%) by 5 years. In a multivariable analysis, acute graft-vs-host disease was associated with increased risk, whereas antiviral prophylaxis was associated with reduced risk for HZ (adjusted hazard ratio, 0.19 [95% CI, .09-.4]). There was no association between CD4+ T-cell counts at 1 year post-CBT and subsequent risk for HZ.
Conclusions: We found a high incidence of HZ after CBT despite antiviral prophylaxis for > 1 year. Based on these findings, we suggest longer duration of prophylaxis for HZ after CBT. Compliance with antiviral prophylaxis, VZV-specific immune monitoring, and vaccination to mitigate HZ after CBT also require further study.
Keywords: antiviral; cord blood; hematopoietic cell transplant; prophylaxis; varicella zoster virus.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- Koc Y, Miller KB, Schenkein DP, et al. . Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant 2000; 6:44–9. - PubMed
-
- Yagi T, Karasuno T, Hasegawa T, et al. . Acute abdomen without cutaneous signs of varicella zoster virus infection as a late complication of allogeneic bone marrow transplantation: importance of empiric therapy with acyclovir. Bone Marrow Transplant 2000; 25:1003–5. - PubMed
-
- Grant RM, Weitzman SS, Sherman CG, Sirkin WL, Petric M, Tellier R. Fulminant disseminated varicella zoster virus infection without skin involvement. J Clin Virol 2002; 24:7–12. - PubMed
-
- Onozawa M, Hashino S, Haseyama Y, et al. . Incidence and risk of postherpetic neuralgia after varicella zoster virus infection in hematopoietic cell transplantation recipients: Hokkaido Hematology Study Group. Biol Blood Marrow Transplant 2009; 15:724–9. - PubMed
-
- Kanbayashi Y, Matsumoto Y, Kuroda J, et al. . Predicting risk factors for varicella zoster virus infection and postherpetic neuralgia after hematopoietic cell transplantation using ordered logistic regression analysis. Ann Hematol 2017; 96:311–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

