Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs
- PMID: 32150268
- PMCID: PMC7063495
- DOI: 10.1001/jamanetworkopen.2020.0423
Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs
Abstract
This cross-sectional study estimates the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs.
Conflict of interest statement
Figures
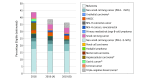
References
-
- American Cancer Society Immune checkpoint inhibitors to treat cancer. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-t.... Published 2018. Accessed January 10, 2010.
-
- US Food and Drug Administration FDA limits the use of Tecentriq and Keytruda for some urothelial cancer patients. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-limit.... Published July 5, 2018. Accessed August 9, 2019.
-
- University of Geneva Public domain antibiotic found highly effective against triple-negative breast cancer. https://medicalxpress.com/news/2019-03-domain-antibiotic-highly-effectiv.... Published March 29, 2019. Accessed August 9, 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

