Self-administered versus provider-administered medical abortion
- PMID: 32150279
- PMCID: PMC7062143
- DOI: 10.1002/14651858.CD013181.pub2
Self-administered versus provider-administered medical abortion
Abstract
Background: The advent of medical abortion has improved access to safe abortion procedures. Medical abortion procedures involve either administering mifepristone followed by misoprostol or a misoprostol-only regimen. The drugs are commonly administered in the presence of clinicians, which is known as provider-administered medical abortion. In self-administered medical abortion, drugs are administered by the woman herself without the supervision of a healthcare provider during at least one stage of the drug protocol. Self-administration of medical abortion has the potential to provide women with control over the abortion process. In settings where there is a shortage of healthcare providers, self-administration may reduce the burden on the health system. However, it remains unclear whether self-administration of medical abortion is effective and safe. It is important to understand whether women can safely and effectively terminate their own pregnancies when having access to accurate and adequate information, high-quality drugs, and facility-based care in case of complications.
Objectives: To compare the effectiveness, safety, and acceptability of self-administered versus provider-administered medical abortion in any setting.
Search methods: We searched Cochrane Central Register of Controlled Trials, MEDLINE in process and other non-indexed citations, Embase, CINAHL, POPLINE, LILACS, ClinicalTrials.gov, WHO ICTRP, and Google Scholar from inception to 10 July 2019.
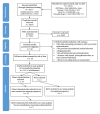
Selection criteria: We included randomized controlled trials (RCTs) and prospective cohort studies with a concurrent comparison group, using study designs that compared medical abortion by self-administered versus provider-administered methods.
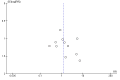
Data collection and analysis: Two reviewers independently extracted the data, and we performed a meta-analysis where appropriate using Review Manager 5. Our primary outcome was successful abortion (effectiveness), defined as complete uterine evacuation without the need for surgical intervention. Ongoing pregnancy (the presence of an intact gestational sac) was our secondary outcome measuring success or effectiveness. We assessed statistical heterogeneity with Chi2 tests and I2 statistics using a cut-off point of P < 0.10 to indicate statistical heterogeneity. Quality assessment of the data used the GRADE approach. We used standard methodological procedures expected by Cochrane.
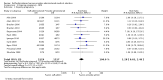
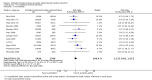
Main results: We identified 18 studies (two RCTs and 16 non-randomized studies (NRSs)) comprising 11,043 women undergoing early medical abortion (≤ 9 weeks gestation) in 10 countries. Sixteen studies took place in low-to-middle income resource settings and two studies were in high-resource settings. One NRS study received analgesics from a pharmaceutical company. Five NRSs and one RCT did not report on funding; nine NRSs received all or partial funding from an anonymous donor. Five NRSs and one RCT received funding from government agencies, private foundations, or non-profit bodies. The intervention in the evidence is predominantly from women taking mifepristone in the presence of a healthcare provider, and subsequently taking misoprostol without healthcare provider supervision (e.g. at home). There is no evidence of a difference in rates of successful abortions between self-administered and provider-administered groups: for two RCTs, risk ratio (RR) 0.99, 95% confidence interval (CI) 0.97 to 1.01; 919 participants; moderate certainty of evidence. There is very low certainty of evidence from 16 NRSs: RR 0.99, 95% CI 0.97 to 1.01; 10,124 participants. For the outcome of ongoing pregnancy there may be little or no difference between the two groups: for one RCT: RR 1.69, 95% CI 0.41 to 7.02; 735 participants; low certainty of evidence; and very low certainty evidence for 11 NRSs: RR 1.28, 95% CI 0.65 to 2.49; 6691 participants. We are uncertain whether there are any differences in complications requiring surgical intervention, since we found no RCTs and evidence from three NRSs was of very low certainty: for three NRSs: RR 2.14, 95% CI 0.80 to 5.71; 2452 participants.
Authors' conclusions: This review shows that self-administering the second stage of early medical abortion procedures is as effective as provider-administered procedures for the outcome of abortion success. There may be no difference for the outcome of ongoing pregnancy, although the evidence for this is uncertain for this outcome. There is very low-certainty evidence for the risk of complications requiring surgical intervention. Data are limited by the scarcity of high-quality research study designs and the presence of risks of bias. This review provides insufficient evidence to determine the safety of self-administration when compared with administering medication in the presence of healthcare provider supervision. Future research should investigate the effectiveness and safety of self-administered medical abortion in the absence of healthcare provider supervision through the entirety of the medical abortion protocol (e.g. during administration of mifepristone or as part of a misoprostol-only regimen) and at later gestational ages (i.e. more than nine weeks). In the absence of any supervision from medical personnel, research is needed to understand how best to inform and support women who choose to self-administer, including when to seek clinical care.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Katherine Gambir does not have any interests to declare.
Kelly Ann Necastro does not have any interests to declare.
Caron Kim does not have any interests to declare.
Bela Ganatra does not have any interests to declare.
Thoai D Ngo does not have any interests to declare.
Figures




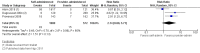





















Update of
- doi: 10.1002/14651858.CD013181
References
References to studies included in this review
Akin 2004 {published and unpublished data}
-
- Akin A, Blum J, Ozalp S, Onderoglu L, Kirca U, Bilgili N, et al. Results and lessons learned from a small medical abortion clinical study in Turkey. Contraception 2004;70(5):401‐6. - PubMed
Alam 2013 {published and unpublished data}
-
- Alam A, Bracken H, Johnston HB, Raghavan S, Islam N, Winikoff B, et al. Acceptability and feasibility of mifepristone‐misoprostol for menstrual regulation in Bangladesh. International Perspectives on Sexual and Reproductive Health 2013;39(2):79‐87. - PubMed
Alam 2018 {published and unpublished data}
-
- Alam A, Lotarevich T, Das TR, Reichenbach L, Bracken H. Mifepristone‐misoprostol for menstrual regulation in public sector facilities in Bangladesh. International Journal of Gynaecology and Obstetrics 2018;140(2):205‐10. - PubMed
Bracken 2006 {published and unpublished data}
-
- Bracken H, Gliozheni O, Kati K, Manoku N, Moisiu R, Shannon C, et al. Mifepristone medical abortion in Albania: results from a pilot clinical research study. European Journal of Contraception & Reproductive Health Care 2006;11(1):38‐46. - PubMed
Bracken 2010 {published and unpublished data}
-
- Bracken H, Family Planning Association of India (FPAI)/Gynuity Health Projects Research Group for Simplifying Medical Abortion in India. Home administration of misoprostol for early medical abortion in India. International Journal of Gynaecology and Obstetrics 2010;108(3):228‐32. - PubMed
Dagousset 2004 {published and unpublished data}
-
- Dagousset I, Fourrier E, Aubeny E, Taurelle R. Use of misoprostol for medical abortion: a trial of the acceptability for home use. Gynécologie, Obstétrique & Fertilité 2004;32(1):28‐33. - PubMed
Elul 2001 ‐ Tunisia {published and unpublished data}
-
- Elul B, Hajri S, Ngoc NN, Ellertson C, Slama CB, Pearlman E, et al. Can women in less‐developed countries use a simplified medical abortion regimen?. Lancet 2001;257(9266):1402‐5. - PubMed
Elul 2001 ‐ Vietnam {published and unpublished data}
-
- Elul B, Hajri S, Ngoc NN, Ellertson C, Slama CB, Pearlman E, et al. Can women in less‐developed countries use a simplified medical abortion regimen?. Lancet 2001;257(9266):1402‐5. - PubMed
Hajri 2004 {published and unpublished data}
-
- Hajri S, Blum J, Gueddana N, Saadi H, Maazoun L, Chelli H, et al. Expanding medical abortion in Tunisia: women's experiences from a multi‐site expansion study. Contraception 2004;70(6):487‐91. - PubMed
Iyengar 2016 {published and unpublished data}
-
- Iyengar K, Klingberg‐Allvin M, Iyengar SD, Paul M, Essen B, Gemzell‐Danielsson K. Home use of misoprostol for early medical abortion in a low resource setting: secondary analysis of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2016;95(2):173‐81. - PubMed
Karki 2009 {published and unpublished data}
-
- Karki C, Pokharel H, Kushwaha A, Manandhar D, Bracken H, Winikoff B. Acceptability and feasibility of medical abortion in Nepal. International Journal of Gynaecology and Obstetrics: the Official Organ of the International Federation of Gynaecology and Obstetrics 2009;106(1):39‐42. - PubMed
Li 2017 {published and unpublished data}
-
- Li CL, Song LP, Tang SY, Zhou LJ, He H, Mo XT, et al. Efficacy, safety, and acceptability of low‐dose mifepristone and self‐administered misoprostol for ultra‐early medical abortion: a randomized controlled trial. Reproductive Sciences 2017;24(5):731‐7. - PubMed
Ngoc 2004 {published and unpublished data}
-
- Ngoc NTN, Nhan VQ, Blum J, Mai TT, Durocher JM, Winikoff B. Is home‐based administration of prostaglandin safe and feasible for medical abortion? Results from a multisite study in Vietnam. BJOG 2004;118(8):814‐9. - PubMed
Okonufua 2014 {published and unpublished data}
-
- Okonofua F, Shittu O, Shochet T, Diop A, Winikoff B. Acceptability and feasibility of medical abortion with mifepristone and misoprostol in Nigeria. International Journal of Gynaecology and Obstetrics 2014;125(1):49‐52. - PubMed
Provansal 2009 {published and unpublished data}
-
- Provansal M, Mimari R, Gregoire B, Agostini A, Thirion X, Gamerre M. Medical abortion at home and at hospital: a trial of efficacy and acceptability. Gynécologie, Obstétrique & Fertilité 2009;37(11‐12):850‐6. - PubMed
Raghavan 2012 {published and unpublished data}
-
- Raghavan S, Ngoc NT, Shochet T, Winikoff B. Clinic‐level introduction of medical abortion in Vietnam. International Journal of Gynaecology and Obstetrics 2012;119(1):39‐43. - PubMed
Shrestha 2014 {published and unpublished data}
-
- Shrestha A, Sedhai LB. A randomized trial of hospital vs home self administration of vaginal misoprostol for medical abortion. Kathmandu University Medical Journal 2014;12(47):185‐9. - PubMed
Shuchita 2008 {published and unpublished data}
-
- Shuchita M, Shveta K, Batya E, Suresh U. Simplifying medical abortion: home administration of misoprostol. Journal of Obstetrics and Gynecology of India 2008;58(5):410‐16.
References to studies excluded from this review
Akin 2005 {published and unpublished data}
-
- Akin A, Kocoglu GO, Akin L. Study supports the introduction of early medical abortion in Turkey. Reproductive Health Matters 2005;13(26):101‐9. - PubMed
Akin 2009 {published and unpublished data}
-
- Akin A, Dabash R, Dilbaz B, Aktun H, Dursun P, Kiran S, et al. Increasing women's choices in medical abortion: a study of misoprostol 400 microg swallowed immediately or held sublingually following 200 mg mifepristone.. European Journal of Contraception & Reproductive Health Care 2009;14(3):169‐75. - PubMed
Arvidsson 2005 {published data only}
-
- Arvidsson C, Hellborg M, Gemzell‐Danielsson K. Preference and acceptability of oral versus vaginal administration of misoprostol in medical abortion with mifepristone. European Journal of Obstetrics & Gynecoloy and Reproductive Biology 2005;123(1):87‐91. - PubMed
Blum 2004 {published data only}
-
- Blum J, Hajri S, Chélli H, Mansour FB, Gueddana N, Winikoff B. The medical abortion experiences of married and unmarried women in Tunis, Tunisia. Contraception 2004;69(1):63‐9. - PubMed
Chong 2015 {published data only}
-
- Chong E, Frye LJ, Castle J, Dean G, Kuehl L, Winikoff B. A prospective, non‐randomized study of home use of mifepristone for medical abortion in the U.S. Contraception 2015;92(3):215‐9. - PubMed
Conkling 2015 {published data only}
-
- Conkling K, Karki C, Tuladhar H, Bracken H, Winikoff B. A prospective open‐label study of home use of mifepristone for medical abortion in Nepal. International Journal of Gynaecology and Obstetrics 2015;128(3):220‐3. - PubMed
Creinin 2007 {published data only}
-
- Creinin MD, Schreiber CA, Bednarek P, Lintu H, Wagner MS, Meyn LA, et al. Mifepristone and misoprostol administered simultaneously versus 24 hours apart for abortion: a randomized controlled trial. Obstetrics and Gynecology 2007;109(4):885‐94. - PubMed
Gaudu 2013 {published data only}
-
- Gaudu S, Crost M, Esterle L. Results of a 4‐year study on 15,447 medical abortions provided by privately practicing general practitioners and gynecologists in France. Contraception 2013;87(1):45‐50. - PubMed
Gonzalez 2001 {published data only}
-
- Gonzalez JA, Carlan SJ, Alverson MW. Outpatient second trimester pregnancy termination. Contraception 2001;63(2):89‐93. - PubMed
Kapp 2006 {published data only}
-
- Kapp N, Borgatta L, Ellis SC, Stubblefield P. Simultaneous very low dose mifepristone and vaginal misoprostol for medical abortion. Contraception 2006;73(5):525‐7. - PubMed
Koh 2017 {published data only}
-
- Koh DSC, Ang EPJ, Coyuco JC, Teo HZ, Huang X, Wei X, et al. Comparing two regimens of intravaginal misoprostol with intravaginal gemeprost for second‐trimester pregnancy termination: a randomised controlled trial. Journal of Family Planning and Reproductive Health Care 2017;43(4):252‐9. - PubMed
Louie 2014 {published data only}
-
- Louie KS, Tsereteli T, Chong E, Aliyeva F, Rzayeva G, Winikoff B. Acceptability and feasibility of mifepristone medical abortion in the early first trimester in Azerbaijan. European Journal of Contraception & Reproductive Health Care 2014;19(6):457‐64. - PubMed
Park 2013 {published data only}
Platais 2016 {published data only}
-
- Platais I, Tsereteli T, Grebennikova G, Lotarevich T, Winikoff B. Prospective study of home use of mifepristone and misoprostol for medical abortion up to 10 weeks of pregnancy in Kazakhstan. International Journal of Gynaecology and Obstetrics 2016;134(3):268‐71. - PubMed
Raghavan 2013 {published data only}
-
- Raghavan S, Tsereteli T, Kamilov A, Kurbanbekova D, Yusupov D, Kasimova F, et al. Acceptability and feasibility of the use of 400 mug of sublingual misoprostol after mifepristone for medical abortion up to 63 days since the last menstrual period: evidence from Uzbekistan. European Journal of Contraception & Reproductive Health Care 2013;18(2):104‐11. - PubMed
Rosen 1984 {published data only}
-
- Rosen AS, Knorring K, Bygdeman M, Christensen NJ. Randomized comparison of prostaglandin treatment in hospital or at home with vacuum aspiration for termination of early pregnancy. Contraception 1984;29(5):423‐35. - PubMed
Swica 2013 {published data only}
-
- Swica Y, Chong E, Middleton T, Prine L, Gold M, Schreiber CA, et al. Acceptability of home use of mifepristone for medical abortion. Contraception 2013;88(1):122‐7. - PubMed
Tsereteli 2016 {published data only}
-
- Tsereteli T, Chong E, Louie K, Bokhua Z, Winikoff B. Acceptability and feasibility of 400mug buccal misoprostol after 200mg mifepristone for early medical abortion in Georgia. Gynuity Health Projects 2016; Vol. 21, issue 5:367‐71. - PubMed
References to ongoing studies
NCT02219100 {published data only}
-
- NCT02219100. Acceptability and feasibility of a demedicalized medical abortion regimen in the Caucasus. https://ClinicalTrials.gov/show/NCT02219100 (first posted 18 August 2014).
NCT02981030 {published data only}
-
- NCT02981030. Acceptability and feasibility of a simplified medical abortion service delivery in Western Ukraine. clinicaltrials.gov/ct2/show/NCT02981030 (first posted 2 December 2016). [NCT02981030]
NCT02985229 {published data only}
-
- NCT02985229. Acceptability and feasibility of medical abortion in Singapore. clinicaltrials.gov/ct2/show/NCT02985229 (first posted 7 December 2016). [NCT02985229]
NCT03346629 {published data only}
-
- NCT03346629. Outpatient service for mid‐trimester termination of pregnancy. clinicaltrials.gov/ct2/show/NCT03346629 (first posted 17 November 2017). [NCT03346629]
NCT03727308 {published and unpublished data}
-
- NCT03727308. Study of clinic‐based versus self‐use of medical abortion pills (MOC). clinicaltrials.gov/ct2/show/NCT03727308 (first posted 1 November 2018). [NCT03727308]
Additional references
Banerjee 2018
-
- Banerjee SK, Kalayanwala S, Manning V, Andersen KL, Raj R, Das A. Is self‐use of medical abortion a viable option?: A systematic review of global evidence with a special focus on India. www.ipasdevelopmentfoundation.org/publications/is‐self‐use‐of‐medical‐ab... 2018.
Bernabé‐Ortiz 2009
Bhalla 2018
-
- Bhalla S, Goyal LD, Bhalla S, Kaur B. Self administered medical abortion pills: evaluation of the clinical outcome and complications among women presenting with unsupervised pill intake to a tertiary care hospital in Malwa Region of Punjab, India. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2018;7(4):1537‐42.
Blum 2012
-
- Blum J, Raghavan S, Dabash R, Ngoc N, Chelli H, Haijri S, et al. Comparison of misoprostol‐only and combined mifepristone‐misoprostol regimens for home‐based early medical abortion in Tunisia and Vietnam. International Journal of Gynaecology and Obstetrics 2012;118:166‐71. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, Accessed prior to 10 January 2020.
Darroch 2011
-
- Darroch J, Singh S. Estimating unintended pregnancies averted from couple‐years of protection (CYP). Guttmacher Institute: www.guttmacher.org/sites/default/files/page_files/guttmacher‐cyp‐memo.pdf 2011.
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Fjersted 2009
Footman 2017
-
- Footman KT, Dijkerman S, Mitu SA, Nuremowla S, Reiss K. Assessing the safety and effectiveness of medical abortion medications purchased from pharmacies: methodological challenges and emerging data. Contraception 2018;97(2):152‐9. - PubMed
Footman 2018a
Footman 2018b
-
- Footman K, LaChance NT. Using evidence to improve quality of pharmacy‐delivered medical abortion. STEP UP case study. London: Marie Stopes International 2018.
Ganatra 2017
Ganle 2019
-
- Ganle JK, Busia NT, Maya E. Availability and prescription of misoprostol for medical abortion in community pharmacies and associated factors in Accra, Ghanna. International Journal of Gynaecology and Obstetrics 2019;144:167‐73. [DOI: ] - PubMed
Giri 2015
-
- Giri A, Srivastav VR, Suwal A, Sharma B. A study of complications following self‐administration with medical abortion pills. Nepal Journal of Obstetrics and Gynaecology 2015;10(1):20‐4.
GRADEpro GDT 2015
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Harper 2007
-
- Harper CC, Blanchard K, Grossman D, Henderson JT, Darney PD. Reducing maternal mortality due to elective abortion: potential impact of misoprostol in low‐resource settings. Internaltion Journal of Gynaecology and Obstetrics 2007;98(1):66‐9. - PubMed
Hendrickson 2016
-
- Hendrickson C, Fetters T, Mupeta S, Vwalllika B, Djemo P, Raisanen K. Client‐pharmacy worker interactions regarding medical abortion in Zambia in 2009 and 2011. International Journal of Gynaecology and Obstetrics 2016;132(2):214‐8. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Huda 2014
-
- Huda FA, Ngo TD, Ahmed A, Alam A, Reichenback L. Availability and provision of misoprostol and other medicines for menstrual regulation among pharmacies in Bangladesh via mystery client survey. International Journal of Gynaecology and Obstetrics 2014;124(2):164‐8. - PubMed
Hyman 2013
-
- Hyman A, Blanchard K, Coeytaux F, Grossman D, Teixeira A. Misoprostol in women's hands: a harm reduction strategy for unsafe abortion. Contraception 2013;87(2):128‐30. - PubMed
Jain 2002
-
- Jain JK, Dutton C, Harwood B, Meckstroth KR, Mishell DR Jr. A prospective randomized, double‐blinded, placebo‐controlled trial comparing mifepristone and vaginal misoprostol to vaginal misoprostol alone for elective termination of early pregnancy. Human Reproduction 2002;17(6):1477‐82. - PubMed
Jejeebhoy 2012
-
- Jejeebhoy SJ, Kalyanwala S, Mundle S, Tank J, Zavier AJ, Kumar R, et al. Feasibility of expanding the medication abortion provider base in India to include ayurvedic physicians and nurses. International Perspectives on Sexual and Reproductive Health 2012;38(3):133–42. - PubMed
Jelinska 2018
-
- Jelinska K, Yanow S. Putting abortion pills into women's hands: realizing the full potential of medical abortion. Contraception 2018;97(2):86‐9. - PubMed
Kahn 2000
-
- Kahn JG, Becker BJ, MacIsaa L, Amory JK, Neuhaus J, Olkin I, et al. The efficacy of medical abortion: a meta‐analysis. Contraception 2000;61:29–40. - PubMed
Kapp 2017
Kero 2009
-
- Kero A, Wulff M, Lalos A. Home abortion implies radical changes for women. European Journal of Contraception & Reproductive Health Care 2009;14(5):324‐33. - PubMed
Kim 2017
Kulier 2011
Langer 2015
-
- Langer A, Meleis A, Knaul FM, Atun R, Aran M, Arreola‐Ornelas H, et al. Women and health: the key for sustainable development. Lancet 2015;386(9999):1165‐210. - PubMed
Lara 2011
-
- Lara D, Garcia SG, Wilson KS, Paz F. How often and under which circumstances do Mexican pharmacy vendors recommend misoprostol to induce an abortion?. International Perspectives on Sexual and Reproductive Heatlh 2011;37(2):75‐83. - PubMed
Lefebvre 2019
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, et al. Chapter 4: Searching for and selecting studies. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019), Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Lokeland 2014
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. - PubMed
Murtagh 2018
-
- Murtagh C, Wells C, Raymond EG, Coeytaux F, Winikoff B. Exploring the feasibility of obtaining mifepristone and misoprostol from the internet. Contraception 2018;97(4):287‐91. - PubMed
Ngo 2011
Olavarrieta 2015
Powell‐Jackson 2015
Raymond 2013
-
- Raymond EG, Shannon C, Weaver MA, Winikoff B. First‐trimester medical abortion with mifepristone 200 mg and misoprostol: a systematic review. Contraception 2013;87(1):26‐37. - PubMed
Raymond 2019
Reiss 2016
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rocca 2018
Rodriguez 2012
-
- Rodriguez MI, Seuc A, Kapp N, Hertzen H, Huong NT, Wojdyla D, et al. Acceptability of misoprostol‐only medical termination of pregnancy compared with vacuum aspiration: an international, multicentre trial. International Journal of Obstetrics and Gynaecology 2012;119(7):817‐23. - PubMed
Ryan 2016
-
- Ryan R, Hill S. How to GRADE the quality of the evidence. CCCG. cccrg.cochrane.org/author‐resources (accessed 10 January 2020).
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Sedgh 2016
Shannon 2008
-
- Shannon C, Winikoff B. How much supervision is necessary for women taking mifepristone and misoprostol for early medical abortion?. Womens Heatlh 2008;4(2):107‐11. - PubMed
Shrestha 2018
-
- Shrestha D, Aryal S, Sharma B. Safety, efficacy and acceptability of early first trimester abortion using oral mifepristone and sublingual misoprostol. Journal of Nepal Health Research Council 2018;16(3):269‐73. - PubMed
Singh 2016
Singh 2018
-
- Singh S, Remez L, Sedgh G, Kwok L, Onda T. Abortion worldwide 2017: uneven progress and unequal access. Guttmacher Institute: www.guttmacher.org/report/abortion‐worldwide‐2017 2018.
Sneeringer 2012
Song 2018
Sterne 2016
Suec 2015
Tamang 2015
-
- Tamang A, Puri M, Lama K, Shrestha P. Pharmacy workers in Nepal can provide the correct information about using mifepristone and misoprostol to women seeking medication to induce abortion. Reproductive Health Matters 2015;22(44 Suppl 1):104‐15. - PubMed
Tamang 2018
-
- Tamang A, Puri M, Masud S, Karki DK, Khadkha D, Singh M, et al. Medical abortion can be provided safely and effectively by pharmacy workers trained within a harm reduction framework: Nepal. Contraception 2018;97(20):137‐43. - PubMed
Tan 2018
-
- Tan YL, Singh K, Tan KH, Gosavi A, Koh D, Abbas D, et al. Acceptability and feasibility of outpatient medical abortion with mefepristone and misoprostol up to 70 days gestation in Singapore. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2018;229:144‐7. - PubMed
Trussell 1999
-
- Trussell J, Ellertson C. Estimating the efficacy of medical abortion. Contraception 1999;60(3):119‐35. - PubMed
Wainwright 2015
-
- Wainwright M, Colvin CJ, Swartz A, Leon N. Self‐management of medical abortion: a qualitative evidence synthesis. Reproductive Health Matters 2016;24(47):155‐67. - PubMed
WHO 2011
-
- World Health Organization. Unsafe abortion. Global and regional estimates of the incidence of unsafe abortion and associated mortality in 2008. www.who.int/reproductivehealth/publications/unsafe_abortion/978924150111... 2011.
WHO 2012
-
- World Health Organization. Safe abortion: technical and policy guidance for health systems, 2nd Edition. www.who.int/reproductivehealth/publications/unsafe_abortion/978924154843... (accessed prior to 28 October 2018).
WHO 2014
-
- World Health Organization. Clinical practice handbook for safe abortion. www.who.int/reproductivehealth/publications/unsafe_abortion/clinical‐pra... (accessed prior to 28 October 2018). - PubMed
WHO 2015
-
- World Health Organization. Health worker roles in providing safe abortion care and post‐abortion contraception. apps.who.int/iris/bitstream/handle/10665/181041/9789241549264_eng.pdf?se... 2015. - PubMed
WHO 2018
-
- World Health Organization. Medical management of abortion. www.who.int/reproductivehealth/guideline‐medical‐abortion‐care/en/ 2018. [Licence: CC BY‐NC‐SA 3.0 IGO] - PubMed
Winikoff 1997
-
- Winikoff B, Sivin I, Coyaji KJ, Cabezas E, Xiao B, Gu S, et al. Safety, efficacy, and acceptability of medical abortion in China, Cuba, and India: a comparative trial of mifepristone‐misoprostol versus surgical abortion. American Journal of Obstetrics and Gynecology 1997;176(2):431‐7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

