Optimising the dose of clonidine to achieve sedation in intensive care unit patients with population pharmacokinetics
- PMID: 32150285
- PMCID: PMC7373711
- DOI: 10.1111/bcp.14273
Optimising the dose of clonidine to achieve sedation in intensive care unit patients with population pharmacokinetics
Abstract
Aims: The aim of this study was to investigate the population pharmacokinetics (PK) of clonidine in intensive care unit (ICU) patients in order to develop a dosing regimen for sedation.
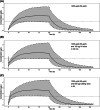
Methods: We included 24 adult mechanically ventilated, sedated patients from a mixed medical and surgical ICU. Intravenous clonidine was added to standard sedation in doses of 600, 1200 or 1800 μg/d. Within each treatment group, 4 patients received a loading dose of half the daily dose administered in 4 hours. Patients gave an average of 12 samples per individual. In total, 286 samples were available for analysis. Model development was conducted with NONMEM and various covariates were tested. After modelling, doses to achieve a target steady-state plasma concentration of >1.5 μg/L were explored using stochastic Monte Carlo simulations for 1000 virtual patients.
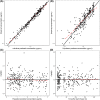
Results: A 2-compartment model was the best fit for the concentration-time data. Clearance (CL) increased linearly with 0.213%/h; using allometric scaling, body weight was a significant covariate on the central volume of distribution (V1). Population PK parameters were: CL 17.1 (L/h), V1 124 (L/70 kg), intercompartmental CL 83.7 (L/h), and peripheral volume of distribution 178 (L), with 33.3% CV interindividual variability on CL and 66.8% CV interindividual variability on V1. Simulations revealed that a maintenance dose of 1200 μg/d provides target sedation concentrations of >1.5 μg/L in 95% of the patients.
Conclusion: A population PK model for clonidine was developed in an adult ICU. A dosing regimen of 1200 μg/d provided a target sedation concentration of >1.5 μg/L.
Keywords: clonidine; population pharmacokinetics; sedation.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures


References
-
- Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21‐26. - PubMed
-
- Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br J Anaesth. 2001;87(5):684‐690. - PubMed
-
- Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86(1):5‐11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

