Concentration-Dependent Seeding as a Strategy for Fabrication of Densely Packed Surface-Mounted Metal-Organic Frameworks (SURMOF) Layers
- PMID: 32150305
- PMCID: PMC7217006
- DOI: 10.1002/chem.202000594
Concentration-Dependent Seeding as a Strategy for Fabrication of Densely Packed Surface-Mounted Metal-Organic Frameworks (SURMOF) Layers
Abstract
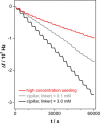
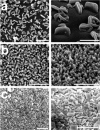
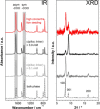
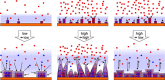
The layer-by-layer (LbL) method is a well-established method for the growth of surface-attached metal-organic frameworks (SURMOFs). Various experimental parameters, such as surface functionalization or temperature, have been identified as essential in the past. In this study, inspired by these recent insights regarding the LbL SURMOF growth mechanism, the impact of reactant solutions concentration on LbL growth of the Cu2 (F4 bdc)2 (dabco) SURMOF (F4 bdc2- =tetrafluorobenzene-1,4-dicarboxylate and dabco=1,4-diazabicyclo-[2.2.2]octane) in situ by using quartz-crystal microbalance and ex situ with a combination of spectroscopic, diffraction and microscopy techniques was investigated. It was found that number, size, and morphology of MOF crystallites are strongly influenced by the reagent concentration. By adjusting the interplay of nucleation and growth, we were able to produce densely packed, yet thin films, which are highly desired for a variety of SURMOF applications.
Keywords: Volmer-Weber growth; layer-by-layer (LbL); metal-organic frameworks; surface chemistry; surface-mounted metal-organic frameworks (SURMOFs).
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kreno L. E., Hupp J. T., Van Duyne R. P., Anal. Chem. 2010, 82, 8042–8046. - PubMed
-
- Heinke L., Tu M., Wannapaiboon S., Fischer R. A., Wöll C., Microporous Mesoporous Mater. 2015, 216, 200–215.
-
- Stassen I., Burtch N., Talin A., Falcaro P., Allendorf M., Ameloot R., Chem. Soc. Rev. 2017, 46, 3185–3241. - PubMed
-
- Mas-Torrent M., Crivillers N., Mugnaini V., Ratera I., Rovira C., Veciana J., J. Mater. Chem. 2009, 19, 1691–1695.
-
- Talin A. A., Centrone A., Ford A. C., Foster M. E., Stavila V., Haney P., Kinney R. A., Szalai V., El Gabaly F., Yoon H. P., Léonard F., Allendorf M. D., Science 2014, 343, 66–69. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

