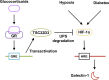
Glucocorticoid-transactivated TSC22D3 attenuates hypoxia- and diabetes-induced Müller glial galectin-1 expression via HIF-1α destabilization
- PMID: 32150332
- PMCID: PMC7176855
- DOI: 10.1111/jcmm.15116
Glucocorticoid-transactivated TSC22D3 attenuates hypoxia- and diabetes-induced Müller glial galectin-1 expression via HIF-1α destabilization
Abstract
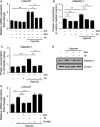
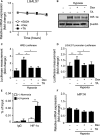
Galectin-1/LGALS1, a newly recognized angiogenic factor, contributes to the pathogenesis of diabetic retinopathy (DR). Recently, we demonstrated that glucocorticoids suppressed an interleukin-1β-driven inflammatory pathway for galectin-1 expression in vitro and in vivo. Here, we show glucocorticoid-mediated inhibitory mechanism against hypoxia-inducible factor (HIF)-1α-involved galectin-1 expression in human Müller glial cells and the retina of diabetic mice. Hypoxia-induced increases in galectin-1/LGALS1 expression and promoter activity were attenuated by dexamethasone and triamcinolone acetonide in vitro. Glucocorticoid application to hypoxia-stimulated cells decreased HIF-1α protein, but not mRNA, together with its DNA-binding activity, while transactivating TSC22 domain family member (TSC22D)3 mRNA and protein expression. Co-immunoprecipitation revealed that glucocorticoid-transactivated TSC22D3 interacted with HIF-1α, leading to degradation of hypoxia-stabilized HIF-1α via the ubiquitin-proteasome pathway. Silencing TSC22D3 reversed glucocorticoid-mediated ubiquitination of HIF-1α and subsequent down-regulation of HIF-1α and galectin-1/LGALS1 levels. Glucocorticoid treatment to mice significantly alleviated diabetes-induced retinal HIF-1α and galectin-1/Lgals1 levels, while increasing TSC22D3 expression. Fibrovascular tissues from patients with proliferative DR demonstrated co-localization of galectin-1 and HIF-1α in glial cells partially positive for TSC22D3. These results indicate that glucocorticoid-transactivated TSC22D3 attenuates hypoxia- and diabetes-induced retinal glial galectin-1/LGALS1 expression via HIF-1α destabilization, highlighting therapeutic implications for DR in the era of anti-vascular endothelial growth factor treatment.
Keywords: HIF-1α; Müller glia; TSC22D3; diabetic retinopathy; galectin-1; glucocorticoid; hypoxia; transactivation.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial or non‐financial interests.
Figures

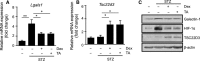

References
-
- Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445‐450. - PubMed
-
- Liu FT, Yang RY, Hsu DK. Galectins in acute and chronic inflammation. Ann N Y Acad Sci. 2012;1253:80‐91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

