A Dose-Ranging Study of Epinephrine Hydrofluroalkane Metered-Dose Inhaler (Primatene® MIST) in Subjects with Intermittent or Mild-to-Moderate Persistent Asthma
- PMID: 32150492
- PMCID: PMC7407001
- DOI: 10.1089/jamp.2019.1558
A Dose-Ranging Study of Epinephrine Hydrofluroalkane Metered-Dose Inhaler (Primatene® MIST) in Subjects with Intermittent or Mild-to-Moderate Persistent Asthma
Abstract
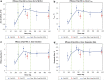
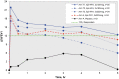
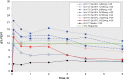
Background: Two sequential single-dose crossover dose-ranging studies were performed to evaluate the clinical efficacy and safety profile of epinephrine hydrofluroalkane (HFA) metered-dose inhaler (MDI) formulation at various doses in subjects with asthma. Methods: In these multicenter, multiarm, double-blinded, or evaluator-blinded studies, subjects were randomized to receive the epinephrine HFA (Primatene® MIST HFA) MDI medication at doses ranging from 90 to 440 μg/dose, as well as to a placebo (PLA) control and an active control of epinephrine CFC (chlorofluorocarbon) MDI (Primatene® MIST CFC) at 220 μg/inhalation. Results: Spirometry testing for FEV1 (Forced Expiratory Volume in one second) demonstrated statistically significant improvements over PLA for epinephrine HFA MDI at all doses above 125 μg, as the amount out of the actuator (i.e., mouthpiece). The efficacy results for epinephrine HFA MDI in the dose range of 125-250 μg were also comparable to epinephrine CFC MDI (220 μg/inh). Safety assessments demonstrated minimal safety concerns for all treatment groups. No notable safety differences were observed between the studied doses of epinephrine HFA MDI and the active control formulation of epinephrine CFC MDI. Conclusion: The findings indicate that epinephrine HFA MDI provided clinically significant bronchodilator efficacy with minimal safety concerns in a dose range of 125-250 μg. These findings confirmed the optimal treatment doses of 125-250 μg that were appropriate for use in longer term 12 and 26 week chronic dosing studies of epinephrine HFA MDI for patients with intermittent or mild to moderate persistent asthma. Clinical trials registration number: NCT01025648.
Keywords: asthma; dose–response; efficacy; inhaled epinephrine; metered-dose inhalers.
Conflict of interest statement
Dr. Edward M. Kerwin served on advisory boards, speaker panels, consultants, or received travel reimbursement from Novartis, AstraZeneca, Amphastar, Forest, Pearl, Sunovion, Teva, Theravance, Mylan, GSK, Boehringer Ingelheim, and Cipla, outside the submitted work. Dr. Donald P. Tashkin reports personal fees from Amphastar Pharmaceuticals, personal fees from AstraZeneca, outside the submitted work. Dr. Thomas R Murphy and Dr. George W. Bensch have no conflicts of interest to disclose. Dr. Jack Y. Zhang, Dr. Mary Z. Luo, and Tony Marrs are employees of Amphastar Pharmaceuticals, Inc. at the time of study and article preparation.
Figures

References
-
- U.S. Food & Drug Administration: Primatene Mist with chlorofluorocarbons no longer available after Dec. 31, 2011. Updated 2011 September 22. https://wayback.archive-it.org/7993/20170112011259/http:/www.fda.gov/For... (Last accessed October16, 2018)
-
- Andrew P: The New York Times. Expected ban on Primatene Mist raises some concernsa. 2016 May 12. www.nytimes.com/2006/05/12/business/12asthma.html?_r=0 (Last accessed June9, 2015)
-
- Mitchell JP, and Nagel MW: Particle size analysis of aerosols from medicinal inhalers. Kona Powder Particle J. 2004;22:32–65
-
- Celestino MT, Magalhães UDO, Fraga AGM, do Carmo FA, Lione V, Castro HC, de Sousa VP, Rodrigues CR, Cabral LM: Rational use of antioxidants in solid oral pharmaceutical preparations. Braz J Pharm Sci. 2012;48:405–415
-
- Primatene® MIST [label]: Armstrong Pharmaceuticals, Inc., Canton, MA. 2018. F5530P

