Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial
- PMID: 32150549
- PMCID: PMC7062239
- DOI: 10.1371/journal.pmed.1003051
Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial
Abstract
Background: There is intense interest about whether modulating gut microbiota can impact systemic metabolism. We investigated the safety of weekly oral fecal microbiota transplantation (FMT) capsules from healthy lean donors and their ability to alter gut microbiota and improve metabolic outcomes in patients with obesity.
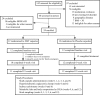
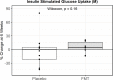
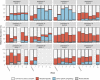
Methods and findings: FMT-TRIM was a 12-week double-blind randomized placebo-controlled pilot trial of oral FMT capsules performed at a single US academic medical center. Between August 2016 and April 2018, we randomized 24 adults with obesity and mild-moderate insulin resistance (homeostatic model assessment of insulin resistance [HOMA-IR] between 2.0 and 8.0) to weekly healthy lean donor FMT versus placebo capsules for 6 weeks. The primary outcome, assessed by intention to treat, was change in insulin sensitivity between 0 and 6 weeks as measured by hyperinsulinemic euglycemic clamps. Additional metabolic parameters were evaluated at 0, 6, and 12 weeks, including HbA1c, body weight, body composition by dual-energy X-ray absorptiometry, and resting energy expenditure by indirect calorimetry. Fecal samples were serially collected and evaluated via 16S V4 rRNA sequencing. Our study population was 71% female, with an average baseline BMI of 38.8 ± 6.7 kg/m2 and 41.3 ± 5.1 kg/m2 in the FMT and placebo groups, respectively. There were no statistically significant improvements in insulin sensitivity in the FMT group compared to the placebo group (+5% ± 12% in FMT group versus -3% ± 32% in placebo group, mean difference 9%, 95% CI -5% to 28%, p = 0.16). There were no statistically significant differences between groups for most of the other secondary metabolic outcomes, including HOMA-IR (mean difference 0.2, 95% CI -0.9 to 0.9, p = 0.96) and body composition (lean mass mean difference -0.1 kg, 95% CI -1.9 to 1.6 kg, p = 0.87; fat mass mean difference 1.2 kg, 95% CI -0.6 to 3.0 kg, p = 0.18), over the 12-week study. We observed variable engraftment of donor bacterial groups among FMT recipients, which persisted throughout the 12-week study. There were no significant differences in adverse events (AEs) (10 versus 5, p = 0.09), and no serious AEs related to FMT. Limitations of this pilot study are the small sample size, inclusion of participants with relatively mild insulin resistance, and lack of concurrent dietary intervention.
Conclusions: Weekly administration of FMT capsules in adults with obesity results in gut microbiota engraftment in most recipients for at least 12 weeks. Despite engraftment, we did not observe clinically significant metabolic effects during the study.
Trial registration: ClinicalTrials.gov NCT02530385.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: EWY has received a research grant from Amgen Inc. and from Doris Duke Charitable Foundation, outside the submitted work. ELH has served as a consultant to Artugen Therapeutics and Matrivax Research and Development Corporation, and has received a research grant from Kaleido, outside the submitted work. CBF, JAB, and MRH are employees of Seres Therapeutics, Inc. All other authors have declared that no competing interests exist.
Figures


Similar articles
-
Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients.Clin Gastroenterol Hepatol. 2020 Apr;18(4):855-863.e2. doi: 10.1016/j.cgh.2019.07.006. Epub 2019 Jul 10. Clin Gastroenterol Hepatol. 2020. PMID: 31301451 Clinical Trial.
-
Recipient microbiome-related features predicting metabolic improvement following fecal microbiota transplantation in adults with severe obesity and metabolic syndrome: a secondary analysis of a phase 2 clinical trial.Gut Microbes. 2024 Jan-Dec;16(1):2345134. doi: 10.1080/19490976.2024.2345134. Epub 2024 Apr 29. Gut Microbes. 2024. PMID: 38685731 Free PMC article. Clinical Trial.
-
Effects of Fecal Microbiome Transfer in Adolescents With Obesity: The Gut Bugs Randomized Controlled Trial.JAMA Netw Open. 2020 Dec 1;3(12):e2030415. doi: 10.1001/jamanetworkopen.2020.30415. JAMA Netw Open. 2020. PMID: 33346848 Free PMC article. Clinical Trial.
-
Fecal microbiota transplantation improves metabolic syndrome parameters: systematic review with meta-analysis based on randomized clinical trials.Nutr Res. 2020 Nov;83:1-14. doi: 10.1016/j.nutres.2020.06.018. Epub 2020 Jul 3. Nutr Res. 2020. PMID: 32987284
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2018 Nov 13;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD012774. doi: 10.1002/14651858.CD012774.pub3. PMID: 30480772 Free PMC article. Updated.
Cited by
-
Gut Microbiota Interventions for the Management of Obesity: A Literature Review.Cureus. 2022 Sep 19;14(9):e29317. doi: 10.7759/cureus.29317. eCollection 2022 Sep. Cureus. 2022. PMID: 36161997 Free PMC article. Review.
-
The Related Metabolic Diseases and Treatments of Obesity.Healthcare (Basel). 2022 Aug 25;10(9):1616. doi: 10.3390/healthcare10091616. Healthcare (Basel). 2022. PMID: 36141228 Free PMC article. Review.
-
Recent insights into the role of microbiome in the pathogenesis of obesity.Therap Adv Gastroenterol. 2022 Aug 9;15:17562848221115320. doi: 10.1177/17562848221115320. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 35967920 Free PMC article. Review.
-
Effect of fecal microbiota transplantation on primary hypertension and the underlying mechanism of gut microbiome restoration: protocol of a randomized, blinded, placebo-controlled study.Trials. 2022 Feb 24;23(1):178. doi: 10.1186/s13063-022-06086-2. Trials. 2022. PMID: 35209934 Free PMC article.
-
Questioning the foundations of the gut microbiota and obesity.Philos Trans R Soc Lond B Biol Sci. 2023 Oct 23;378(1888):20220221. doi: 10.1098/rstb.2022.0221. Epub 2023 Sep 4. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37661739 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

