Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry
- PMID: 32150576
- PMCID: PMC7082060
- DOI: 10.1371/journal.ppat.1008392
Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry
Abstract
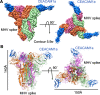
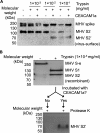
Coronaviruses recognize a variety of receptors using different domains of their envelope-anchored spike protein. How these diverse receptor recognition patterns affect viral entry is unknown. Mouse hepatitis coronavirus (MHV) is the only known coronavirus that uses the N-terminal domain (NTD) of its spike to recognize a protein receptor, CEACAM1a. Here we determined the cryo-EM structure of MHV spike complexed with mouse CEACAM1a. The trimeric spike contains three receptor-binding S1 heads sitting on top of a trimeric membrane-fusion S2 stalk. Three receptor molecules bind to the sides of the spike trimer, where three NTDs are located. Receptor binding induces structural changes in the spike, weakening the interactions between S1 and S2. Using protease sensitivity and negative-stain EM analyses, we further showed that after protease treatment of the spike, receptor binding facilitated the dissociation of S1 from S2, allowing S2 to transition from pre-fusion to post-fusion conformation. Together these results reveal a new role of receptor binding in MHV entry: in addition to its well-characterized role in viral attachment to host cells, receptor binding also induces the conformational change of the spike and hence the fusion of viral and host membranes. Our study provides new mechanistic insight into coronavirus entry and highlights the diverse entry mechanisms used by different viruses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

