Model-Informed Drug Development for Everolimus Dosing Selection in Pediatric Infant Patients
- PMID: 32150661
- PMCID: PMC7180003
- DOI: 10.1002/psp4.12502
Model-Informed Drug Development for Everolimus Dosing Selection in Pediatric Infant Patients
Abstract
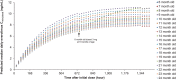
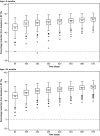
Everolimus is currently approved in Europe as an adjunctive therapy for patients aged ≥ 2 years with tuberous sclerosis complex (TSC)-associated treatment-refractory partial-onset seizures, based on the EXIST-3 study (NCT01713946) results. As TSC-associated seizures can also affect children aged between 6 months and 2 years, a modeling and simulation (M&S) approach was undertaken to extrapolate exposure (trough plasma concentration (Cmin )) after a dose of 6 mg/m2 and reduction in seizure frequency (RSF). A physiologically based pharmacokinetic model using Simcyp was developed to predict Cmin in adult and pediatric patients, which was then used by a population pharmacodynamic model and a linear mixed effect model to predict short-term and long-term efficacy in adults (for validation) and in children, respectively. Based on the results of the M&S study, everolimus at the dose of 6 mg/m2 is anticipated to be an efficacious treatment in children 6 months to 2 years of age (up to 77.8% RSF) with concentrations within the recommended target range.
© 2020 Novartis Pharmaceutical Company. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are or were employees of Novartis at the time of the research. None of the authors declares any conflict of interest.
Figures



References
-
- Crino, P.B. , Nathanson, K.L. & Henske, E.P. The tuberous sclerosis complex. N Engl. J. Med. 355, 1345–1356 (2006). - PubMed
-
- Overwater, I.E. et al Epilepsy in children with tuberous sclerosis complex: chance of remission and response to antiepileptic drugs. Epilepsia 56, 1239–1245 (2015). - PubMed
-
- Curatolo, P. , Jozwiak, S. & Nabbout, R. Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur. J. Paediatric Neurol. 16, 582–586 (2012). - PubMed
-
- Chen, X. et al Dual inhibition of PI3K and mTOR mitigates compensatory AKT activation and improves tamoxifen response in breast cancer. Mol. Cancer Res. 11, 1269–1278 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

