Pyruvate alleviates high glucose-induced endoplasmic reticulum stress and apoptosis in HK-2 cells
- PMID: 32150786
- PMCID: PMC7193158
- DOI: 10.1002/2211-5463.12834
Pyruvate alleviates high glucose-induced endoplasmic reticulum stress and apoptosis in HK-2 cells
Abstract
Endoplasmic reticulum (ER) stress plays a critical role in the development of diabetic nephropathy (DN). We previously demonstrated that pyruvate (Pyr)-enriched oral rehydration solution improved glucometabolic disorders and ameliorated DN outcome in db/db mice. Here, we investigated the effects of Pyr on high glucose-induced ER stress and apoptosis in HK-2 cells. Our results suggest that high glucose can induce reactive oxygen species production, apoptosis and ER stress in HK-2 cells, and that Pyr treatment can ameliorate these effects and restore the expression of key proteins involved in ER stress. Thus, Pyr may have potential for the development of novel strategies for the prevention and treatment of clinical DN.
Keywords: HK-2 cells; apoptosis; diabetes; diabetic nephropathy; endoplasmic reticulum stress; pyruvate.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

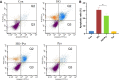
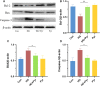

References
-
- Ju Y, Su Y, Chen Q, Ma K, Ji T, Wang Z, Li W and Li W (2019) Protective effects of Astragaloside IV on endoplasmic reticulum stress‐induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother 109, 84–92. - PubMed
-
- Dejeans N, Barroso K, Fernandez‐Zapico ME, Samali A and Chevet E (2015) Novel roles of the unfolded protein response in the control of tumor development and aggressiveness. Semin Cancer Biol 33, 67–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

