In Vivo Positive Magnetic Resonance Imaging Applications of Poly(methyl vinyl ether-alt-maleic acid)-coated Ultra-small Paramagnetic Gadolinium Oxide Nanoparticles
- PMID: 32150823
- PMCID: PMC7179159
- DOI: 10.3390/molecules25051159
In Vivo Positive Magnetic Resonance Imaging Applications of Poly(methyl vinyl ether-alt-maleic acid)-coated Ultra-small Paramagnetic Gadolinium Oxide Nanoparticles
Abstract
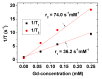
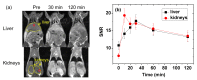
The study of ultra-small paramagnetic gadolinium oxide (Gd2O3) nanoparticles (NPs) as in vivo positive (T1) magnetic resonance imaging (MRI) contrast agents is one of the most attractive fields in nanomedicine. The performance of the Gd2O3 NP imaging agents depends on the surface-coating materials. In this study, poly(methyl vinyl ether-alt-maleic acid) (PMVEMA) was used as a surface-coating polymer. The PMVEMA-coated paramagnetic ultra-small Gd2O3 NPs with an average particle diameter of 1.9 nm were synthesized using the one-pot polyol method. They exhibited excellent colloidal stability in water and good biocompatibility. They also showed a very high longitudinal water proton spin relaxivity (r1) value of 36.2 s-1mM-1 (r2/r1 = 2.0; r2 = transverse water proton spin relaxivity) under a 3.0 tesla MR field which is approximately 10 times higher than the r1 values of commercial molecular contrast agents. High positive contrast enhancements were observed in in vivo T1 MR images after intravenous administration of the NP solution sample, demonstrating its potential as a T1 MRI contrast agent.
Keywords: T1 magnetic resonance imaging; contrast agent; paramagnetic; poly (methyl vinyl ether-alt-maleic acid); ultra-small Gd2O3 nanoparticle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hydrophilic Biocompatible Poly(Acrylic Acid-co-Maleic Acid) Polymer as a Surface-Coating Ligand of Ultrasmall Gd2O3 Nanoparticles to Obtain a High r1 Value and T1 MR Images.Diagnostics (Basel). 2020 Dec 22;11(1):2. doi: 10.3390/diagnostics11010002. Diagnostics (Basel). 2020. PMID: 33375089 Free PMC article.
-
Ligand-size dependent water proton relaxivities in ultrasmall gadolinium oxide nanoparticles and in vivo T1 MR images in a 1.5 T MR field.Phys Chem Chem Phys. 2014 Oct 7;16(37):19866-73. doi: 10.1039/c4cp01946f. Phys Chem Chem Phys. 2014. PMID: 25123195
-
Gadolinium (III) oxide nanoparticles coated with folic acid-functionalized poly(β-cyclodextrin-co-pentetic acid) as a biocompatible targeted nano-contrast agent for cancer diagnostic: in vitro and in vivo studies.MAGMA. 2019 Aug;32(4):487-500. doi: 10.1007/s10334-019-00738-2. Epub 2019 Feb 7. MAGMA. 2019. PMID: 30730021
-
Ultrasmall Europium, Gadolinium, and Dysprosium Oxide Nanoparticles: Polyol Synthesis, Properties, and Biomedical Imaging Applications.Mini Rev Med Chem. 2020;20(17):1767-1780. doi: 10.2174/1389557520666200604163452. Mini Rev Med Chem. 2020. PMID: 32496986 Review.
-
Contrast agents: magnetic resonance.Handb Exp Pharmacol. 2008;(185 Pt 1):135-65. doi: 10.1007/978-3-540-72718-7_7. Handb Exp Pharmacol. 2008. PMID: 18626802 Review.
Cited by
-
Enhanced Tumor Imaging Using Glucosamine-Conjugated Polyacrylic Acid-Coated Ultrasmall Gadolinium Oxide Nanoparticles in Magnetic Resonance Imaging.Int J Mol Sci. 2022 Feb 4;23(3):1792. doi: 10.3390/ijms23031792. Int J Mol Sci. 2022. PMID: 35163714 Free PMC article.
-
Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine.Pharmaceuticals (Basel). 2025 Jan 24;18(2):154. doi: 10.3390/ph18020154. Pharmaceuticals (Basel). 2025. PMID: 40005968 Free PMC article. Review.
-
Polymer-coated hexagonal upconverting nanoparticles: chemical stability and cytotoxicity.Front Chem. 2023 Jun 23;11:1207984. doi: 10.3389/fchem.2023.1207984. eCollection 2023. Front Chem. 2023. PMID: 37426333 Free PMC article.
-
Magnetic Nanoparticle-Based High-Performance Positive and Negative Magnetic Resonance Imaging Contrast Agents.Pharmaceutics. 2023 Jun 15;15(6):1745. doi: 10.3390/pharmaceutics15061745. Pharmaceutics. 2023. PMID: 37376193 Free PMC article. Review.
-
Synthesis, Characterizations, and 9.4 Tesla T2 MR Images of Polyacrylic Acid-Coated Terbium(III) and Holmium(III) Oxide Nanoparticles.Nanomaterials (Basel). 2021 May 20;11(5):1355. doi: 10.3390/nano11051355. Nanomaterials (Basel). 2021. PMID: 34065511 Free PMC article.
References
-
- Atabaev T.S., Lee J.H., Han D.-W., Kim H.K., Hwang Y.-H. Fabrication of carbon coated gadolinia particles for dual-mode magnetic resonance and fluorescence imaging. J. Adv. Ceram. 2015;4:118–122. doi: 10.1007/s40145-015-0139-z. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

