PINK1/Parkin Mediated Mitophagy, Ca2+ Signalling, and ER-Mitochondria Contacts in Parkinson's Disease
- PMID: 32150829
- PMCID: PMC7084677
- DOI: 10.3390/ijms21051772
PINK1/Parkin Mediated Mitophagy, Ca2+ Signalling, and ER-Mitochondria Contacts in Parkinson's Disease
Abstract
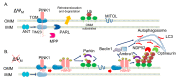
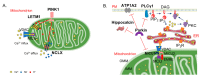
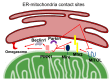
Endoplasmic reticulum (ER)-mitochondria contact sites are critical structures for cellular function. They are implicated in a plethora of cellular processes, including Ca2+ signalling and mitophagy, the selective degradation of damaged mitochondria. Phosphatase and tensin homolog (PTEN)-induced kinase (PINK) and Parkin proteins, whose mutations are associated with familial forms of Parkinson's disease, are two of the best characterized mitophagy players. They accumulate at ER-mitochondria contact sites and modulate organelles crosstalk. Alterations in ER-mitochondria tethering are a common hallmark of many neurodegenerative diseases including Parkinson's disease. Here, we summarize the current knowledge on the involvement of PINK1 and Parkin at the ER-mitochondria contact sites and their role in the modulation of Ca2+ signalling and mitophagy.
Keywords: Ca2+; ER–mitochondria tethering; PINK1; Parkin; mitophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Karabiyik C., Lee M.J., Rubinsztein D.C. Autophagy impairment in Parkinson’s disease. Essays Biochem. 2017;61:711–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

