The Photoreceptor Components FaWC1 and FaWC2 of Fusarium asiaticum Cooperatively Regulate Light Responses but Play Independent Roles in Virulence Expression
- PMID: 32150839
- PMCID: PMC7143034
- DOI: 10.3390/microorganisms8030365
The Photoreceptor Components FaWC1 and FaWC2 of Fusarium asiaticum Cooperatively Regulate Light Responses but Play Independent Roles in Virulence Expression
Abstract
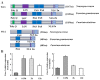
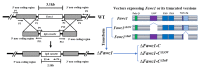
Fusarium asiaticum belongs to one of the phylogenetical subgroups of the F. graminearum species complex and is epidemically predominant in the East Asia area. The life cycle of F. asiaticum is significantly regulated by light. In this study, the fungal blue light receptor white collar complex (WCC), including FaWC1 and FaWC2, were characterized in F. asiaticum. The knockout mutants ΔFawc1 and ΔFawc2 were generated by replacing the target genes via homologous recombination events. The two mutants showed similar defects in light-induced carotenoid biosynthesis, UV-C resistance, sexual fruiting body development, and the expression of the light-responsive marker genes, while in contrast, all these light responses were characteristics in wild-type (WT) and their complementation strains, indicating that FaWC1 and FaWC2 are involved in the light sensing of F. asiaticum. Unexpectedly, however, the functions of Fawc1 and Fawc2 diverged in regulating virulence, as the ΔFawc1 was avirulent to the tested host plant materials, but ΔFawc2 was equivalent to WT in virulence. Moreover, functional analysis of FaWC1 by partial disruption revealed that its light-oxygen-voltage (LOV) domain was required for light sensing but dispensable for virulence, and its Zinc-finger domain was required for virulence expression but not for light signal transduction. Collectively, these results suggest that the conserved fungal blue light receptor WCC not only endows F. asiaticum with light-sensing ability to achieve adaptation to environment, but it also regulates virulence expression by the individual component FaWC1 in a light-independent manner, and the latter function opens a way for investigating the pathogenicity mechanisms of this important crop disease agent.
Keywords: Fusarium asiaticum; White collar complex; photobiology; transcription factor; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cuomo C.A., Güldener U., Xu J.R., Trail F., Turgeon B.G., Pietro A.D., Walton J.D., Ma L.J., Baker S.E., Rep M., et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science. 2007;317:1400–1402. doi: 10.1126/science.1143708. - DOI - PubMed
-
- O’Donnell K., Ward T.J., Geiser D.M., Kistler H.C., Aoki T. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 2004;41:600–623. doi: 10.1016/j.fgb.2004.03.003. - DOI - PubMed
-
- O’Donnell K., Ward T.J., Aberra D., Kistler H.C., Aoki T., Orwig N., Kimura M., Bjørnstad Å., Klemsdal S.S. Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genet. Biol. 2008;45:1514–1522. doi: 10.1016/j.fgb.2008.09.002. - DOI - PubMed
-
- Starkey D.E., Ward T.J., Aoki T., Gale L.R., Kistler H.C., Geiser D.M., Suga H., Tóth B., Varga J., O’Donnell K. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet. Biol. 2007;44:1191–1204. doi: 10.1016/j.fgb.2007.03.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

