Engineering Microneedles for Therapy and Diagnosis: A Survey
- PMID: 32150866
- PMCID: PMC7143426
- DOI: 10.3390/mi11030271
Engineering Microneedles for Therapy and Diagnosis: A Survey
Abstract
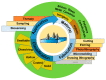
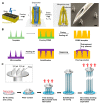
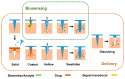
Microneedle (MN) technology is a rising star in the point-of-care (POC) field, which has gained increasing attention from scientists and clinics. MN-based POC devices show great potential for detecting various analytes of clinical interests and transdermal drug delivery in a minimally invasive manner owing to MNs' micro-size sharp tips and ease of use. This review aims to go through the recent achievements in MN-based devices by investigating the selection of materials, fabrication techniques, classification, and application, respectively. We further highlight critical aspects of MN platforms for transdermal biofluids extraction, diagnosis, and drug delivery assisted disease therapy. Moreover, multifunctional MNs for stimulus-responsive drug delivery systems were discussed, which show incredible potential for accurate and efficient disease treatment in dynamic environments for a long period of time. In addition, we also discuss the remaining challenges and emerging trend of MN-based POC devices from the bench to the bedside.
Keywords: diagnosis; drug delivery; microneedle; point of care.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Razmi N., Hasanzadeh M. Current advancement on diagnosis of ovarian cancer using biosensing of CA 125 biomarker: Analytical approaches. TrAC Trends Anal. Chem. 2018;108:1–12. doi: 10.1016/j.trac.2018.08.017. - DOI
-
- Shafiee A., Ghadiri E., Kassis J., Pourhabibi Zarandi N., Atala A. Biosensing Technologies for Medical Applications, Manufacturing, and Regenerative Medicine. Curr. Stem Cell Rep. 2018;4:105–115. doi: 10.1007/s40778-018-0123-y. - DOI
-
- Markets and Markets. Wearable Medical Devices Market by Device (Diagnostic (Heart, Pulse, BP, Sleep), Therapeutic (Pain, Insulin, Rehabilitation)), Application (Sport, Fitness, RPM), Type (Smartwatch, Patch), Distribution Channel (Pharmacy, Online) - Global Forecast to 2022. [(accessed on 1 March 2020)]; Available online: https://www.marketsandmarkets.com/Market-Reports/wearable-medical-device....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

