Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals
- PMID: 32150899
- PMCID: PMC7150973
- DOI: 10.3390/pharmaceutics12030229
Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals
Abstract
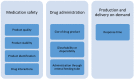
The utilization of three-dimensional (3D) printing technologies as innovative manufacturing methods for drug products has recently gained growing interest. From a technological viewpoint, proof-of-concept on the performance of different printing methods already exist, followed by visions about future applications in hospital or community pharmacies. The main objective of this study was to investigate the perceptions of healthcare professionals in a tertiary university hospital about oral 3D-printed medicines for pediatric patients by means of focus group discussions. In general, the healthcare professionals considered many positive aspects and opportunities in 3D printing of pharmaceuticals. A precise dose as well as personalized doses and dosage forms were some of the advantages mentioned by the participants. Especially in cases of polypharmacy, incorporating several drug substances into one product to produce a polypill, personalized regarding both the combination of drug substances and the doses, would benefit drug treatments of several medical conditions and would improve adherence to medications. In addition to the positive aspects, concerns and prerequisites for the adoption of 3D printing technologies at hospital settings were also expressed. These perspectives are suggested by the authors to be focus points for future research on personalized 3D-printed drug products.
Keywords: 3D printing; 3D-printed medicines; children; focus group study; healthcare professionals; hospital pharmacy; pediatrics; personalized medication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Goyanes A., Madla C.M., Umerji A., Duran Piñeiro G., Giraldez Montero J.M., Lamas Diaz M.J., Gonzalez Barcia M., Taherali F., Sánchez-Pintos P., Couce M.-L., et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019;567:118497. doi: 10.1016/j.ijpharm.2019.118497. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources